Synthesis of 1,1′-Azobis(3,5-dinitropyrazole)
LI Ya-nan, YU Tao, LIAN Peng, LI Xiang-zhi, WANG Bo-zhou
(Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
1 Introduction
New strategies in energetic materials research focus on nitrogen-rich/high positive heat of formation compounds[1-2]. Nitrogen-rich compounds have therefore received increasing attention as promising candidates for high energy-density materials(HEDM) which may be used as propellants, explosives or especially as gas generators[3-5], and the generation of nitrogen gas as an end product of nitrogen-rich compounds is highly favored for the enhancement of energy and avoiding environmental pollution[6-8]. Nitrogen-rich compounds based on C/N heteroaromatic rings with high nitrogen content like triazole, tetrazole, triazine and tetrazine are at the forefront of high energy research. Recently, the combination of an azo group with nitrogen-rich heteroaromatic rings has been extensively studied because the azo linkage not only desensitizes but also dramatically increases the heat of formation , such as 3,3′-azobis(1,2,4-triazole)[9], 5,5′-dinitro-3,3′-azo-1H-1,2,4-triazole[10], 3,3′-azobis-(6-amino-1,2,4,5-tetrazine)[11]and 4,4′,6,6′-tetra(azido)azo-1,3,5-triazine[12], where the two heteroaromatic rings are connected by a C—NN—C linkage. However, if the azo group is attached to the nitrogen of the heteroaromatic rings to create a rather long chain of catenated nitrogens (N—NN—N linkage), such a polynitrogen structure can result in unique properties and features of these energetic compounds, such as 4,4′-azo-1,2,4-triazole[9], 1,1′-azobis-1,2,3-triazole[13], 1,1′-azobis(tetrazole)[1], 2,2′-azobis(5-nitrotetrazole)[14]and 1,1′-azobis(5-methyltetrazole)[15]. Unfortunately, some of the above compounds don′t own excellent detonating performance or thermal unstable.
In this paper, a nitrogen-rich energetic material, 1,1′-azobis(3,5-dinitropyrazole)(ABDNP), which contain the NO2, N—NN—N and pyrazole ring, was synthesized using 3,5-dinitropyrazole(DNP) as starting material(Scheme 1). The property of ABDNP was estimated by a B3LYP method on 6-31G(d,p) basis set of Gaussian 09[16]procedure.
2 Experimental
2.1 General methods and materials
Melting points were determined using an open capillary tube. Infrared(IR) spectra were recorded on a Nicolet NEXUS 870 Infrared spectrometer using KBr pellets.1H NMR and13C NMR spectra were recorded on a Bruker AV 500 NMR spectrometer with TMS as the internal standard. Mass spectra were acquired using a GCMS-QP 2010 Micromass UK spectrometer. Elemental analyses were performed on a VARI-EI-3 elemental an alyzer.
DNP andO-mesitylenesulfonylhydroxylamine(MSH) were self-synthesized. Ethanol, potassium hydroxide, acetonitrile and isopropyl were of AR grade, purchased from Chengdu Kelong Chemical Reagents Factory.
2.2 Synthetic route
ABDNP was synthesized using DNP and MSH as starting materials. The synthetic route was as follow (Scheme 1).
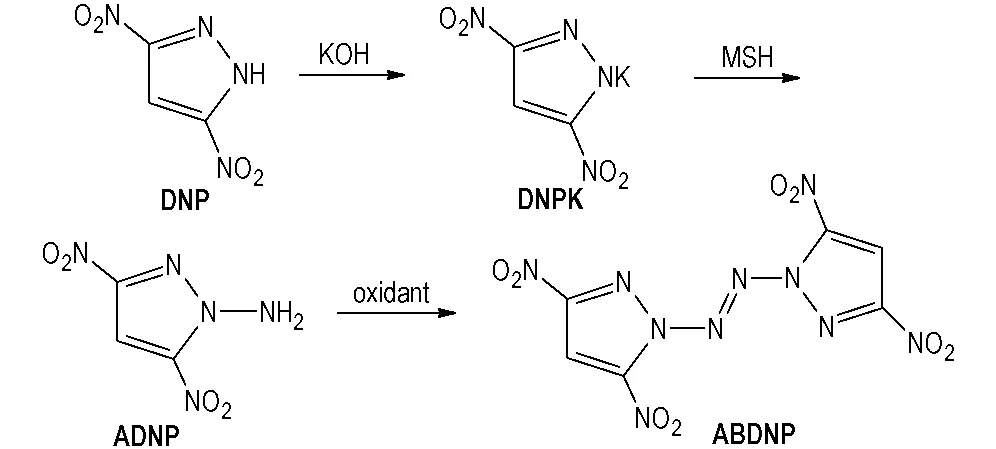
Scheme 1 Synthetic route of ABDNP
2.3 Synthesis of ABDNP
2.3.1 Synthesis of 1-amino-3,5-dinitropyrazole(ADNP)
2.0 g(0.13 mol) of DNP was dissolved in 15.0 mL ethanolat room temperature, then 14.2 g 5% potassium hydroxide(0.13 mol) was added dropwise at 5-10 ℃. After two hours at room temperature, the precipitate was filtered off, washed with ethanol and ice-water, and dried to give 2.25 g yellow solid 3,5-dinitropyrazole potassium salt(DNPK) with a yield of 90.7%.1H NMR(D2O-d2, 500 MHz),δ:7.399(s, 1H, —CH);13C NMR(D2O-d2, 125 MHz),δ: 99.24, 156.18; IR(KBr,ν/cm-1): 3157(—CH), 1539, 1378, 1351(—NO2), 1270, 835, 755(Pyrazole). Anal. Calcd for C3HN4O4K(%): C 18.37, H 0.51, N 28.56; found(%) C 18.40, H 0.49, N 28.54.
0.5 g(2.55 mmol) of DNPK was dissolved in 15.0 mL anhydrous acetonitrileat room temperature. Then a solution of 0.77 g(3.57 mmol) of MSH in 2.5 mL anhydrous acetonitrile was added dropwise to the above reaction mixture at 0-3 ℃. After 20 h at room temperature, the precipitate was filtered off, the filtrate was concentrated in vacuo to give yellow oil compound. Recrystallization with water to obtain 0.37 g buff solid ADNP with a yield of 84.1%, m. p.: 111.5-112.1 ℃.1H NMR(DMSO-d6, 500 MHz),δ: 7.105(s, H, —CH), 8.006(s, 2H, —NH2);13C NMR(DMSO-d6, 125 MHz),δ: 101.69, 140.07, 147.66; IR(KBr,ν/cm-1): 3355, 3287(—NH2), 3154(—CH), 1508, 1381, 1356(—NO2), 1556, 845, 741(Pyrazole). Anal. Calcd for C3H3N5O4(%): C 20.82, H 1.75, N 40.46; found(%) C 20.80, H 1.78, N 40.50.
2.3.2 Synthesis of ABDNP
0.1 g(0.58 mmol) of ADNP was dissolved in 10.0 mL acetonitrileat room temperature, cooled to 0-5℃, with stirring under nitrogen, and treated with 0.082 g(0.75 mmol) oft-butyl hypochlorite over 3 minutes. The resulting solution was stirred at 20-25 ℃ for another 2 hours, concentrated in vacuo, and triturated with 2.0 mL isopropyl alcohol. The precipitate was filtered off, washed with isopropyl alcohol and ice-water, and dried to give 43.0 mg yellow solid ABDNP with a yield of 43.5%.1H NMR(DMSO-d6, 500 MHz),δ: 8.625(s, 2H, 2-CH);13C NMR(DMSO-d6, 125 MHz),δ: 106.03, 146.09, 153.39; IR(KBr,ν/cm-1): 3154, 3135(—CH), 1530, 1390, 1331(—NO2), 1215, 1136, 1027(pyrazole ring); Anal. Calcd for C6H2N10O8(%): C 21.06, H 0.59, N 40.94; found(%) C 21.08, H 0.57, N 40.92. ESI-MS(m/z): 377[M+35Cl]-、379[M+37Cl]-.
3 Results and discussion
3.1 Synthesis
3.1.1 Synthesis of MSH
MSH is unfortunately not stable in storage so before each reaction it was prepared from ethylO-2,4,6-trimethylsulphonylacetohydroximate by hydrolysis with 70% perchloric acid. After hydrolysis it was poured into ice-water, extracted with dichloromethane, and the dichloromethane solution was dried over sodium sulfate, the dichloromethane was concentrated in vacuo to give white solid compound MSH.
3.1.2 Synthesis of ABDNP
To obtain ABDNP, we treated ADNP with different oxidizing agents as azo coupling reagent by following the synthetic procedures used for the generation of reported compounds[1,9,13-15]. A host of different oxidants, which were successfully used to prepare compounds, containing the C—NN—C structure from energetic compounds with the C—NH2group, were selected. Unfortunately, no formation of compound ABDNP was observed by following use of oxidants except tert-butyl hypochlorite(t-BuOCl), sodium dichloroisocyanurate(SDCI) and trichloroisocyanuricacid(TCICA) in Table 1.
Table 1 Oxidative coupling reaction of ADNP with different oxidants
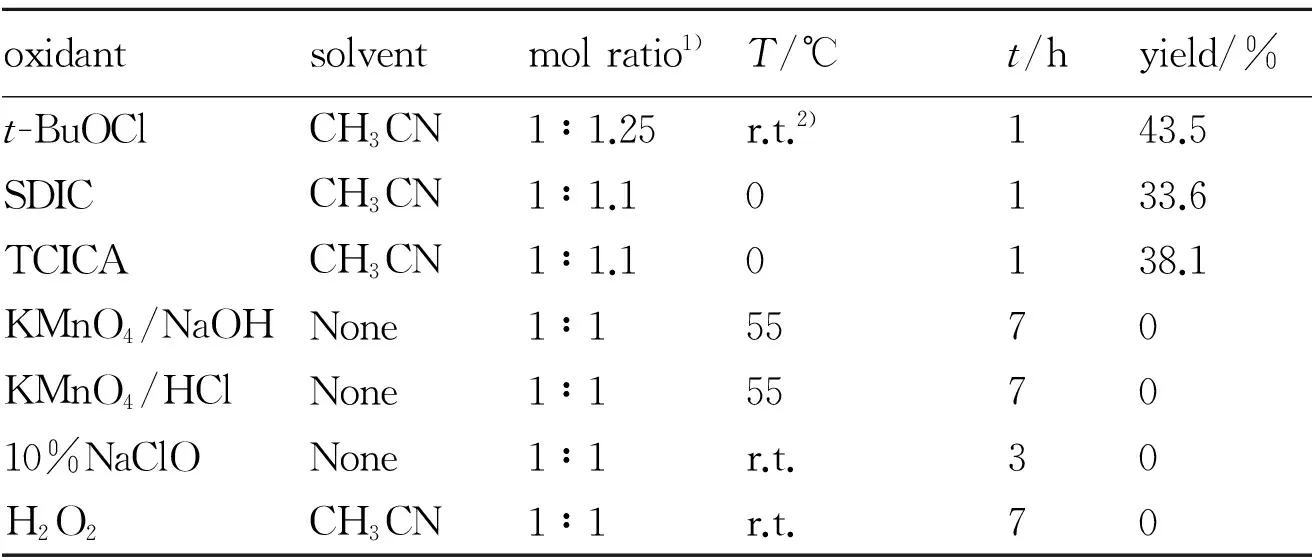
oxidantsolventmolratio1)T/℃t/hyield/%t-BuOClCH3CN1∶1.25r.t.2)143.5SDICCH3CN1∶1.10133.6TCICACH3CN1∶1.10138.1KMnO4/NaOHNone1∶15570KMnO4/HClNone1∶1557010%NaClONone1∶1r.t.30H2O2CH3CN1∶1r.t.70
Note: 1) mol ratio of 1-amino-3,5-dinitropyrazole to oxidant, 2) room temperature.
3.2 Property of ABDNP
The structure of ABDNP was optimized by Gaussian 09[16]in order to obtain their stable geometric configuration, and their density and heat of formation were computed. The detonation parameters were obtained by VLW equation[17]using density and heat of formation as basic data. The performance of ABDNP and 1,3,5-trinitro-1,3,5-triazinane(RDX)[18]were shown in Table 2.
Table 2 Properties of ABDNP and RDX
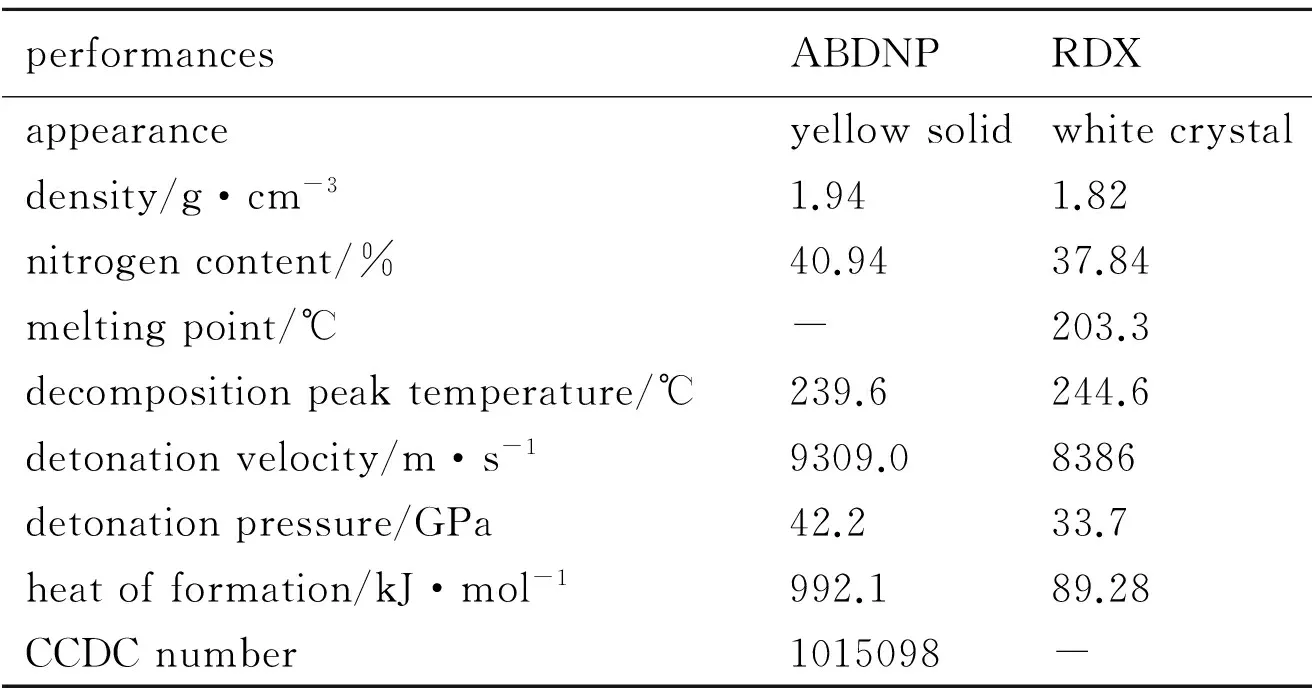
performancesABDNPRDXappearanceyellowsolidwhitecrystaldensity/g·cm-31.941.82nitrogencontent/%40.9437.84meltingpoint/℃-203.3decompositionpeaktemperature/℃239.6244.6detonationvelocity/m·s-19309.08386detonationpressure/GPa42.233.7heatofformation/kJ·mol-1992.189.28CCDCnumber1015098-
4 Conclusions
A nitrogen-rich, polynitro energetic compound with anN,N-azo linkage, 1,1′-azobis(3,5- dinitropyrazole)(ABDNP), can be obtained by three different coupling reagents from 1-amino-3,5-dinitropyrazole(ADNP) with yield of 43.5%.
ABDNP has better detonation performances than RDX. Especially, ABDNP has higher positive heat of formation, which is 992.1 kJ·mol-1than 89.28 kJ·mol-1of RDX. The thermal decomposition temperature of ABDNP is 239.6 ℃, indicating that ABDNP has preferable thermal stability.
[1] Klap?tke T M, Piercey D G. 1,1′-Azobis(tetrazole): a highly energetic nitrogen-rich compound with a N10chain[J].InorgChem, 2011, 50(7): 2732-2734.
[2] LI Ya-nan, ZHANG Zhi-zhong, GE Zhong-xue, et al. Study of furoxan derivatives for energetic applications[J].ChinJChem, 2013, 31: 520-524.
[3] Zhang Y, Huang Y, Parrish D A, et al. 4-Amino-3,5-dinitropyrazolate salts—highly insensitive materials[J].JMaterChem, 2011, 21: 6891-6897.
[4] Fischer N, Izsák D, Klap?tke T M, et al. Nitrogen-rich 5,5′-bistetrazolates and their potential use in propellant systems: a comprehensive study[J].Chem-EurJ, 2012, 18: 4051-4062.
[5] Dippold A A, Klap?tke T M, Martin F A, et al. Nitraminoazoles based on ANTA—a comprehensive study of structural and energetic properties[J].EurJInorgChem, 2012, 14: 2429-2443
[6] Wang R, Guo Y, Zeng Z, et al. Nitrogen-rich nitroguanidyl-functionalized tetrazolate energetic salts[J].ChemCommun, 2009, 3: 2697-2699.
[7] Fendt T, Fischer N, Klap?tke T M, et al. N-rich salts of 2-methyl-5-nitraminotetrazole: secondary explosives with low sensitivities[J].InorgChem, 2011, 50: 1447-1458.
[8] G?bel M, Karaghiosoff K, Klap?tke T M, et al. Nitrotetrazolate-2N-oxides and the strategy ofN-oxide introduction[J].JAmChemSoc, 2010, 132: 17216-17226.
[9] QI Cai, LI Sheng-hua, LI Yu-chuan, et al. A novel stable high-nitrogen energetic material: 4,4′-azobis(1,2,4-triazole)[J].JMaterChem, 2011, 21: 3221-3225.
[10] Naud D L, Hiskey M A, Harry H H. Synthesis and explosive properties of 5,5′-dinitro-3,3′-azo-1H-1,2,4- triazole[J].JEnergMater, 2003, 21: 57-62.
[11] Chavez D E, Hiskey M A, Gilardi R D. 3,3′-Azobis(6-amino-1,2,4,5-tetrazine): a novel high-nitrogen energetic material[J].AngewChemIntEd, 2000, 39: 1791-1793.
[12] Huynh M H V, Hiskey M A, Hartline E L, et al. Polyazido high-nitrogen compounds: hydrazo- and azo-1,3,5-triazine[J].AngewChemIntEd, 2004, 43: 4924-4928.
[13] LI Yu-chun, QI Cai, LI Sheng-hua, et al. 1,1′-Azobis-1,2,3-triazole: a high-nitrogen compound with stable N8structure and photochromism[J].JAmChemSoc, 2010, 132: 12172-12173.
[14] Klap?tke T M, Piercey D G, Stierstorfer J. Amination of energetic anions: high-performing energetic materials[J].DaltonTrans, 2012, 41: 9451-9459.
[15] TANG Yong-xing, YANG Hong-wei, SHEN Jian-hua, et al. Synthesis and characterization of 1,1′-azobis(5- methyltetrazole)[J].NewJChem, 2012, 36: 2447-2450.
[16] Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09[CP], Gaussian, Inc, Wallingford CT, 2009.
[17] WU Xiong, LONG Xin-ping, HE Bi, et al. The VLW equation of state for detonation products[J].ScienceinChina, 2008, 38(12):1129-1131.
[18] HE Zhi-wei, LIU Zu-liang, WANG Ai-ling. Influence of 2,6-diamino-3,5-dinitropyridine-1-oxide on properties of RDX[J].ChinJExplosPropel, 2010, 33(1): 11-14.

