Solitary Langerhans cell histiocytosis of frontal lobe: a case report and literature review
Shanshan Cai, Sheng Zhang, Xueyong Liu, Yuanxiang Lin, Chunlin Wu, Yupeng Chen, Jianping Hu, Xingfu Wang
1Department of Pathology, the Second Affiliated Hospital of Fujian Medical University, Quanzhou 362000, China;2Department of Pathology,3Department of Neurosurgery,4Department of Radiology, the First Affliated Hospital of Fujian Medical University, Fuzhou 350005, China
Correspondence to: Xingfu Wang. Department of Pathology, the First Affliated Hospital of Fujian Medical University, Fuzhou 350005, China.
Email: wangxfu@gmail.com.
Solitary Langerhans cell histiocytosis of frontal lobe: a case report and literature review
Shanshan Cai1, Sheng Zhang2, Xueyong Liu2, Yuanxiang Lin3, Chunlin Wu1, Yupeng Chen2, Jianping Hu4, Xingfu Wang2
1Department of Pathology, the Second Affiliated Hospital of Fujian Medical University, Quanzhou 362000, China;2Department of Pathology,3Department of Neurosurgery,4Department of Radiology, the First Affliated Hospital of Fujian Medical University, Fuzhou 350005, China
Correspondence to: Xingfu Wang. Department of Pathology, the First Affliated Hospital of Fujian Medical University, Fuzhou 350005, China.
Email: wangxfu@gmail.com.
The brain parenchymal Langerhans cell histiocytosis (LCH) without systemic disease or lytic skull lesions is extremely rare. We report a 23-year-old male presenting with new onset 1 hour seizure with loss of consciousness 20 days prior to admission, and recurrent seizure 2 weeks later. Brain magnetic resonance imaging (MRI) showed an irregularly mass with enhancement involving the right frontal lobe. Microscopically, the lesion was characterized by sheets of Langerhans cells in addition to reactive infammatory elements. Immunohistochemically, Langerhans cells were positive for Langerin, CD1a and S-100 protein. The patient received no chemotherapy or radiotherapy after surgery. After 24 months of follow-up, no recurrence or other systemic lesions were observed. Although there is no standard treatment for solitary cerebral LCH, the prognosis generally appears to be good.
Langerhans cell histiocytosis (LCH); histiocytosis; immunohistochemistry; pathology
View this article at:http://www.thecjcr.org/article/view/3700/4578
Introduction
Langerhans cell histiocytosis (LCH), characterized by clonal proliferation of Langerhans cells and focal aggregates of variable numbers of eosinophils, lymphocytes, neutrophils, foamy histiocytes and multinucleated giant cells, is a rare systemic disease that occurs frequently in children (1). LCH tends to involve the skeletons and surrounding soft tissue, presenting with osteolytic lesions. Unifocal central nervous system (CNS) involvement without systemic disease or lytic skull lesions is extremely rare. We report a case of solitary LCH of the right frontal lobe without osseous involvement, and review the relevant literatures.
Case report
A 23-year-old male presented with new onset 1 hour seizure with loss of consciousness 20 days prior to admission, and recurrent seizure 2 weeks later. The physical and neurological examinations were normal. His medical history was unremarkable. No hereditary syndromes were disclosed. Brain magnetic resonance imaging (MRI) showed an irregularly mass of 4.1 cm X 3.1 cm X 3.5 cm involving the right frontal lobe, hypo-/iso-intense on T1-weighted image (Figure 1A), iso-/hyper-intense on T2-weighted image (Figure 1B) and fluid-attenuated inversion recovery (FLAIR). After the administration of intravenous contrast gadolinium-diethylene triamine pentaacetic acid (DTPA), the lesion showed moderate to intense homogeneous enhancement (Figure 1C).
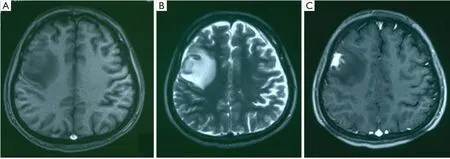
Figure 1 (A) Unenhanced MRI showed a slightly hypointense mass with surrounding hypointense edema on T1-weighted imaging; (B) and a slightly hyperintense nodular lesion approaching to the surface of the brain on T2-weighted imaging; (C) contrast enhancement T1-weighted MR imaging showed the nodular lesion enhanced obviously.
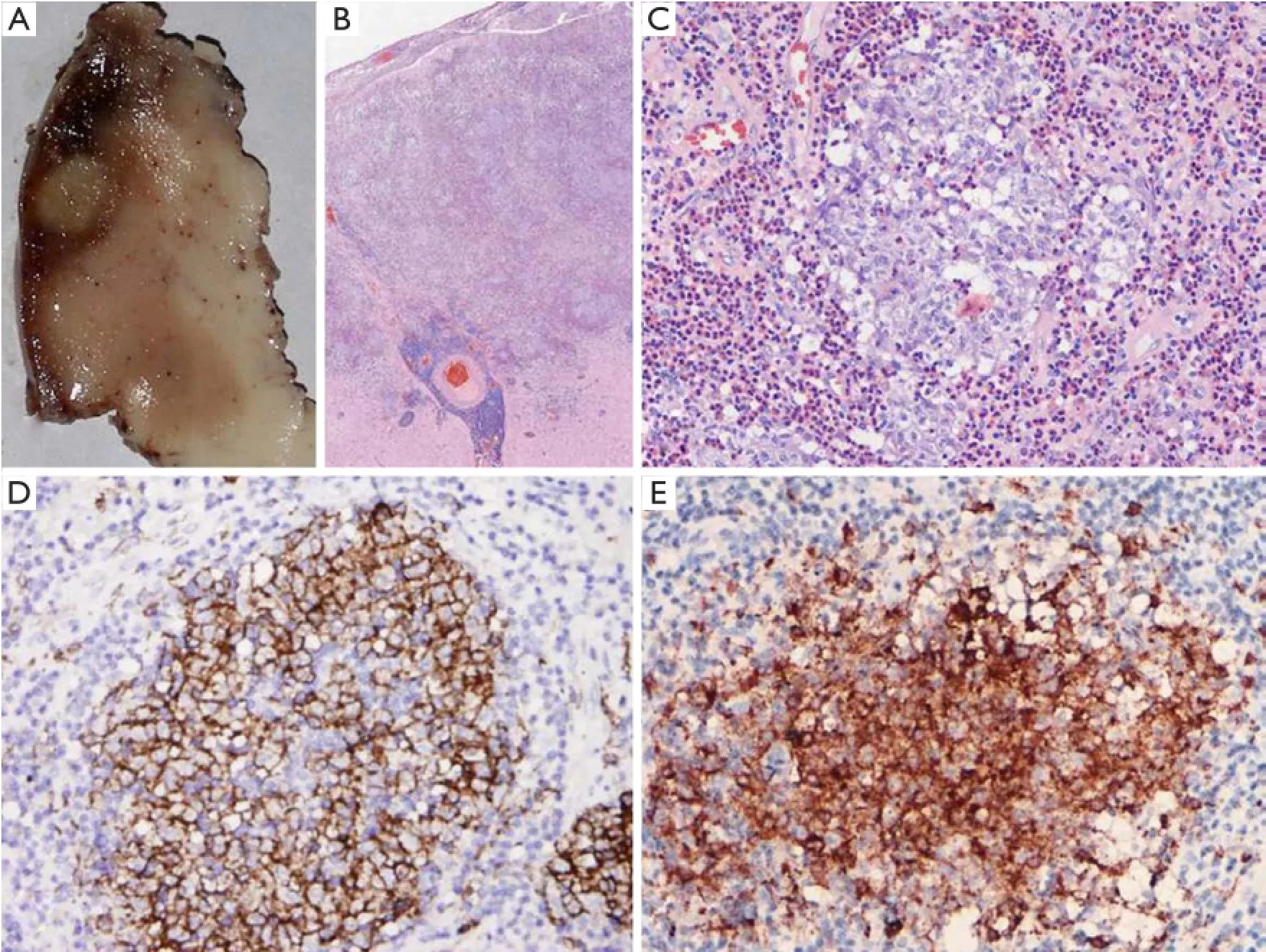
Figure 2 Grossly, the cross-section revealed a well-defined gray-white nodular lesion measuring 0.8 cm X 0.6 cm in grey matter (A). Microscopically, the lesion was limited to the grey matter. It is composed of sheets of Langerhans cells and reactive elements including eosinophils, neutrophils, macrophages, small lymphocytes, plasma cells, and so on (B, H&E, X40) (C, H&E, X400). Immunohistochemically, Langerhans cells were positive for CD1a (D) and Langerin (E).
A right frontal craniotomy was performed under general anesthesia on January 14, 2012. The lesion was located in the pallium of the precentral gyrus. The mass lesion was totally resected and sent for pathological evaluation. Grossly, the cross-section revealed a well-defned nodular lesion measuring 0.8 cm X 0.6 cm in grey matter, with gray-white and slightly tough appearance (Figure 2A). Microscopically, the lesion was limited to the grey matter. It was characterized by sheets of histiocyte-like cells in addition to reactive elements,including eosinophils, neutrophils, macrophages, small lymphocytes, plasma cells, and occasional multinucleated giant cells. The proliferating histiocytes were mostly large and round without signifcant atypia, and had abundant clearto-eosinophilic cytoplasm and indented or convoluted nuclei with linear condensation of chromatin, resulting in a coffee bean appearance of nuclei (Figure 2B,C). Mitosis and necrosis were absent. The other characteristic finding was a reactive inflammatory infiltrate, with a prominence of eosinophils. Immunohistochemically, the histiocyte-like cells were positive for CD1a (Figure 2D), Langerin (Figure 2E) and S-100 protein, which confrmed their origination from Langerhans cells. Glial fibrillary acidic protein (GFAP), synaptophysin (SYN), epithelial membrane antigen (EMA), cytokeratin (CK) and vimentin were negative for Langerhans cells. The final diagnosis was LCH. The patient received no chemotherapy or radiotherapy after surgery. After 24 months of follow-up, no recurrence or other systemic lesions were observed.
Discussion
Isolated involvement of CNS without evidence of systemic disease and concomitant osseous involvement is very rare (2). Only 21 cases of solitary LCH involving brain parenchyma have been reported (3-22). Including the present one, we summarized all the 22 cases in our report. They consisted of 15 males and 7 females with a mean age of 28.5 years (range, 3-40 years). The fndings suggested that the cerebral LCH tends to occur later than that of other systems. The temporal lobe was involved most commonly, followed by the frontal lobe and the parietal lobe.
Clinical manifestations of LCH varied with location and may also include nonspecific symptoms of mass effect. Headaches (21 of 22 cases), seizures (19 of 22 cases), and localizing signs, such as hemiparesis and sensory deficits, were most common. Intracerebral solitary LCH had no characteristic neuroimaging patterns. These lesions generally revealed as low-density masses with surrounding edema on CT, and occasional homogeneous contrast enhancement. MRI was variable in the cases reported. Some cases showed low- or iso-intensity on T1-weighted imaging and hyperintensity lesions on T2-weighted imaging without gadolinium contrast enhancement, while the others demonstrated hyperintensity on T2-weighted imaging with marked homogeneous enhancement after administration of gadolinium (23).
The final diagnosis should be made by pathological features. Histologically, the typical morphology of LCH appears to have a variable number of Langerhans cells accompanied by reactive elements (2). In most cases, some Langerhans cells show the typical cytological features of indented or convoluted nuclei with linear condensation of chromatin, resulting in a coffee bean appearance of nuclei. The nuclear features are diagnostic, which may be used for distinguishing Langerhans cells from other types of histiocytes. Co-expression of CD1a and Langerin is most commonly used to confrm the diagnosis, and the positivity for S-100 protein is also helpful. Although ultrastructural demonstration of Birbeck granules is of great diagnostic relevance in the definition of this tumor type, Birbeck granules just exist in 2-69% of Langerhans cells (20,24). The brain parenchymal LCH often requires a broader category of differential diagnosis, including granulomatous diseases such as sarcoidosis, tuberculosis, non-LCH, histiocytosarcoma and lymphoma.
Optimal treatment for LCH with CNS mass lesions is not yet well defined. Ng Wing Tin et al. suggested that vinblastine, with or without steroids, could potentially be a useful therapeutic option in LCH with CNS mass lesions, especially for those with inoperable lesions or multiple lesions (25). Dhall et al. found that 2-chlorodeoxyadenosine is an active agent in patients with CNS LCH, with the possible exception of neurodegenerative disease (26). However, the preferred treatment for unifocal CNS LCH is surgical resection. Sometimes radiation and chemotherapy may be needed due to incompletely resection, especially in children. Along with the important role of chemokines in the pathogenesis of LCH has been gradually recognized, the agents aiming at cytokine, such as TNF-α, inhibition, are used to block the development process of LCH, which is expected to be the foundation of targeted therapy (27). Of all the 22 patients, 2 cases of recurrence have been reported, and one of them died from the disease (18). Recently, Laurencikas et al. reported that about one fourth of children with LCH had an MRI signature consistent with some degree of neurodegeneration. The finding highlighted the need for long-term follow up in patients with LCH-associated CNS neurodegeneration (28). Totally, the prognosis of solitary cerebral LCH generally appears to be good.
Acknowledgements
Disclosure: The authors declare no confict of interest.
1. Willman CL, Busque L, Griffth BB, et al. Langerhans'-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N Engl J Med 1994;331:154-60.
2. Grois N, Prayer D, Prosch H, et al. Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain 2005;128:829-38.
3. Sivalingam S, Corkill G, Ellis WG, et al. Focal eosinophilic granuloma of the temporal lobe. Case report. J Neurosurg 1977;47:941-5.
4. Cerdá-Nicolas M, Broseta J, Peydrò-Olaya A, et al. Primary eosinophilic granuloma of the frontal lobe. Virchows Arch A Pathol Anat Histol 1980;388:221-8.
5. Moscinski LC, Kleinschmidt-DeMasters BK. Primary eosinophilic granuloma of frontal lobe. Diagnostic use of S-100 protein. Cancer 1985;56:284-8.
6. Greenwood SM, Martin JS, Towfghi J. Unifocal eosinophilic granuloma of the temporal lobe. Surg Neurol 1982;17:441-4.
7. Waldron RL 2nd, Paysinger BD, Reynolds JC, et al. Histiocytosis-X: extra-hypothalamic involvement of the central nervous system. Br J Radiol 1984;57:435-8.
8. Hammar S, Weaver RA, Keranen VJ. Left temporal lobe cerebral cortex mass in a 19-year-old male. Ultrastruct Pathol 1986;10:583-91.
9. Penar PL, Kim JH, Chyatte D. Solitary eosinophilic granuloma of the frontal lobe. Neurosurgery 1987;21:566-8.
10. Itoh H, Waga S, Kojima T, et al. Solitary eosinophilic granuloma in the frontal lobe: case report. Neurosurgery 1992;30:295-8.
11. Eriksen B, Janinis J, Variakojis D, et al. Primary histiocytosis X of the parieto-occipital lobe. Hum Pathol 1988;19:611-4.
12. Caresio JF, McMillan JH, Batnitzky S. Coexistent intraand extracranial mass lesions: an unusual manifestations of histiocytosis X. AJNR Am J Neuroradiol 1991;12:82.
13. Breidahl WH, Ives FJ, Khangure MS. Cerebral and brain stem Langerhans cell histiocytosis. Neuroradiology 1993;35:349-51.
14. Montine TJ, Hollensead SC, Ellis WG, et al. Solitary eosinophilic granuloma of the temporal lobe: a case report and long-term follow-up of previously reported cases. Clin Neuropathol 1994;13:225-8.
15. Grant GA, Kim DK, Shaw CM, et al. Solitary eosinophilic granuloma of the temporal lobe: case report and review of the literature. Brain Tumor Pathol 1999;16:55-9.
16. Marafoti T, Cardia E. Solitary eosinophilic granuloma of cerebral lobes. Value of immunohistochemistry for a diagnostic interpretation. Zentralbl Pathol 1994;140:391-6.
17. Bogaert J, Verschakelen JA, d'Haen B, et al. Diagnosis of atypical intracerebral Langerhans cell histiocytosis suggested by concomitant lung abnormalities. A case report. Rofo 1994;161:369-71.
18. Vital A, Loiseau H, Kantor G, et al. Primary Langerhans' cell histiocytosis of the central nervous system with fatal outcome. Case report. J Neurosurg 1996;85:1156-60.
19. Bergmann M, Yuan Y, Brück W, et al. Solitary Langerhans cell histiocytosis lesion of the parieto-occipital lobe: a case report and review of the literature. Clin Neurol Neurosurg 1997;99:50-5.
20. Rodríguez-Pereira C, Borrás-Moreno JM, Pesudo-Martínez JV, et al. Cerebral solitary Langerhans cell histiocytosis: report of two cases and review of the literature. Br J Neurosurg 2005;19:192-7.
21. Katati MJ, Martin JM, Pastor J, et al. Isolated primary Langerhans' cell histiocytosis of central nervous system. Neurocirugia (Astur) 2002;13:477-8.
22. Reznik M, Stevenaert A, Bex V, et al. Focal brain invasion as the frst manifestation of Langerhans cell histiocytosis in an adult. Case report. Clin Neuropathol 1993;12:179-83.
23. Prayer D, Grois N, Prosch H, et al. MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis. AJNR Am J Neuroradiol 2004;25:880-91.
24. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol 2008;25:291-5.
25. Ng Wing Tin S, Martin-Duverneuil N, Idbaih A, et al. Effcacy of vinblastine in central nervous system Langerhans cell histiocytosis: a nationwide retrospective study. Orphanet J Rare Dis 2011;6:83.
26. Dhall G, Finlay JL, Dunkel IJ, et al. Analysis of outcome for patients with mass lesions of the central nervous system due to Langerhans cell histiocytosis treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer 2008;50:72-9.
27. Donadieu J, Chalard F, Jeziorski E. Medical management of langerhans cell histiocytosis from diagnosis to treatment. Expert Opin Pharmacother 2012;13:1309-22.
28. Laurencikas E, Gavhed D, St?lemark H, et al. Incidence and pattern of radiological central nervous system Langerhans cell histiocytosis in children: a population based study. Pediatr Blood Cancer 2011;56:250-7.
Cite this article as:Cai S, Zhang S, Liu X, Lin Y, Wu C, Chen Y, Hu J, Wang X. Solitary Langerhans cell histiocytosis of frontal lobe: a case report and literature review. Chin J Cancer Res 2014;26(2):211-214. doi: 10.3978/j.issn.1000-9604.2014.02.12
10.3978/j.issn.1000-9604.2014.02.12
Submitted Jan 06, 2014. Accepted for publication Feb 14, 2014.
 Chinese Journal of Cancer Research2014年2期
Chinese Journal of Cancer Research2014年2期
- Chinese Journal of Cancer Research的其它文章
- Highlight of Endoscopic procedures developed in Japan
- The changing world of drug development
- Solitary rib metastasis of nasopharyngeal carcinoma
- Multiple primary malignancies: a report of two cases
- Huang's three-step maneuver for laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer
- Antitumor effects of artesunate on human breast carcinoma MCF-7 cells and IGF-IR expression in nude mice xenografts
