A critical quality parameter in quantitative fused-core chromatography: The injection volume
Jente Boonen, Mtthis D'hondt, Lieselotte Veryser, Kthelijne Peremns,Christin Burvenih, Brt De Spiegeleer,*
aDrug Quality and Registration (DruQuaR) Group, Faculty of Pharmaceutical Sciences, Ghent University, Harelbekestraat 72,B-9000 Ghent, Belgium
bDepartment of Veterinary Medical Imaging and Small Animal Orthopaedics, Faculty of Veterinary Medicine, Ghent University,Salisburylaan 133, B-9820 Merelbeke, Belgium
cDepartment of Comparative Physiology and Biometrics, Faculty of Veterinary Medicine, Ghent University, Salisburylaan 133,B-9820 Merelbeke, Belgium
Available online 17 February 2013
1. Introduction
The demand for high-throughput high performance liquid chromatography (HPLC) systems is ever-growing and rapid low-cost analyses for drug screening and discovery, drug development and quality control become more desirable.Therefore, newly developed fused-core HPLC stationary phases such as HALO?columns have attracted the interest of the chromatographic community [1-3]. Due to their small particle size (2.7 μm) and unique particle technology with a 0.5 μm porous superficial shell fused to a solid inert core(1.7 μm), these columns allow for fast and high-performance separations when compared to the conventional, fully porous,packed columns [4-7].
Like sub-2-μm ultra high performance liquid chromatography (UHPLC) particles, fused-core columns generate flatter van Deemter curves, with the additional advantage of lower back pressure[8].Consequently,higher flow rates,resulting in faster LC separations with almost no significant loss in chromatographic performance, can be used [9,10]. Although the separation efficiency is generally somewhat lower than those of sub-2-μm UPLC columns, conventional standard HPLC equipment can be used with fused-core columns [11].However, using the conventional HPLC equipment, several extra-column operational aspects are becoming critical in fused-core chromatography. Extra-column dispersion (ECD)is mainly affected by extra-column volumes (ECV), which is expressed by four additive sources: the injection volume, the volume of the pre- and post-column connecting tube, detector flow cell and signal processing electronics (like detector time and sample rate) [1]. Alexander et al. [1] investigated the influence of aforementioned volumetric parameters extensively, except for the injection volume.
We hypothesize that due to ECV, developmental parameters like the injection volume are also becoming more pronounced in fused-core compared to conventional HPLC chromatography and should not be neglected. Therefore, we have concentrated on the effects of altering the injection volume using fused-core chromatography on conventional HPLC equipment and standard HPLC operational settings.The pharmaceutically interesting plant bioactive spilanthol was chosen as the model compound, as this N-alkylamide is included in one of our laboratory research topics [12,13].
To demonstrate the applicability of the injection volume optimized fused-core HPLC method,the transdermal behavior of spilanthol in hydroxypropyl methyl cellulose (HPMC)-based patches containing different percentages of plasticizer triethyl citrate (TEC) (5% and 20%, m/m) and penetration enhancer oleic acid (OA) (0 and 5%, m/m) was investigated using human skin in a Franz diffusion cell (FDC) set-up.
2. Materials and methods
2.1. Reagents
2.2. Method description
A superficially porous HALO?RP-amide column(4.6 mm ×50 mm, 2.7 μm particle size, 90 ?A pore size, and 150 m2/g specific surface area),protected by an RP-amide guard column(4.6 mm × 5 mm, 2.7 μm particle size) (both from Advanced Material Technology, Wilmington, DE, USA) was used.According to the quality assurance report of the manufacturer,the minimal number of theoretical plates(Ncol)is 10,000.From the t0of uracil (0.2 min) at a flow rate of 1.8 mL/min,the void volume (Vcol) is calculated to be 0.360 mL.
A newly purchased column and aged column (i.e., having approximately 1000 injections with similar matrices)were used with a degassed isocratic mobile phase, consisting of 0.1% or 1% (v/v) formic acid in MeOH/H2O (70/30, v/v) or 1% (v/v)formic acid in ACN/H2O(60/40,v/v).The flow rate was set at 1.5 mL/min. UV detection was performed at 237 nm. Dilutions of the A. Vogel Spilanthes extract in PBS as well as in 70/30 (v/v) MeOH/H2O were made to accommodate injection volumes ranging from 2 to 100 μL, while maintaining consistent mass loads (0.499 μg). For each condition, triplicate injections were made.The five chromatographic characteristics(retention time, height, area, theoretical plates and symmetry factor) were calculated in accordance with the European Pharmacopoeia: the theoretical plates (N) were determined from peak widths at half height and the symmetry factor (As)was calculated at one-twentieth of the peak height from the ratio of the whole width and two times the distance between the peak maximum and leading edge of the peak [14].
2.3. HPLC instrumentation
The HPLC apparatus consisted of a Waters Alliance 2695 separation module equipped with an autosample injector and a Waters 2996 PDA controlled by Empower 2 software(all Waters,Millford,USA).Injection accuracy has been stated by the supplier as±2% (50 μL,n=6)with a range of 0.1-100 μL.The standard instrumental configurations for conventional HPLC were used without further optimization.Connective pre-column tubing(from auto-injector valve outlet to column inlet) consisted of a 135 mm(0.3 mm i.d.) stainless steel tubing and a 460 mm (0.25 mm i.d.)peek tubing. For post-column (from column outlet to flow cell inlet),a 400 mm(0.12 mm i.d.)peek tubing was used.The detector cell volume was 9.3 μL. Considering the dimensions of the RPamide fused-core column and the capacity factor k′for the described method (k′=5), the maximum acceptable detector cell volume (Vcell) [1] is not exceeded and thus appropriate:
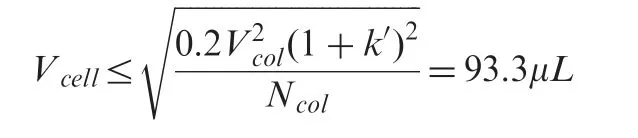
The equations on maximum acceptable detector time constant(τ)and minimum detector sampling rate[1]were used as a guidance for detector configurations.
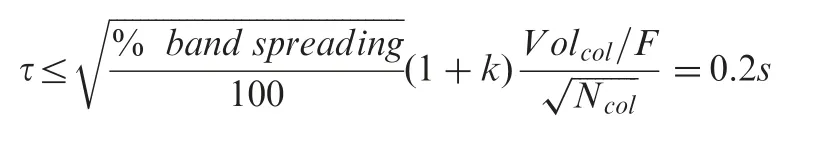
If maximal band spreading of 5% is considered, using the 4.6 mm × 50 mm RP-amide fused-core column with a flow rate F of 1.5 mL/min,the time constant τ should be equal to or smaller than 0.2 s. A detector time constant of 0.2 s was selected.
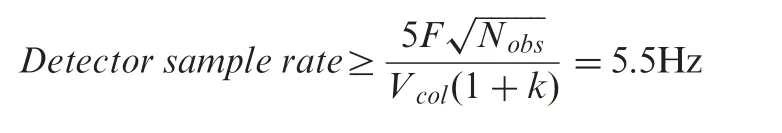
Considering a maximal 10% overall loss in efficiency due to extra-column band broadening (i.e., Nobs=0.9Ncol) and the column and method parameters as mentioned above,the required detector sampling rate should be ≥5.5 Hz.Although the standard setting of 10 Hz for conventional separations is still relatively low for fused-core chromatography,this frequency is typically used in conventional HPLC and hence was applied in our experiments.
2.4. Franz diffusion cell experiments using human skin
The target concentration of the HPMC-based patches was 8%(m/m) spilanthol per dry weight. Appropriate amounts of concentrated Jambu extract, HPMC, TEC and OA were dissolved in a 10 mL chloroform:methanol:dichloromethane(4:4:2, v/v/v) mixture to obtain four different HPMC patches,containing 5%TEC/ 0%OA, 5%TEC/ 5%OA, 20%TEC/0%OA and 20%TEC/ 5%OA (m/m), respectively. The obtained solutions were shaken vigorously and poured on silicone paper. After solvent-evaporation overnight, patches were stored in a desiccator for one day. Before transdermal application, the patches were punched (diameter=0.95 cm),weighed and their thickness(32±1 μm,mean±SEM,n=36)was determined using a micrometer (Heidenhain, Schaumburg, USA). The permeation of spilanthol through human skin was determined using static Franz diffusion cells (FCDs)as described elsewhere [12,13]. The thickness of the skin,obtained from two female patients (55 and 77 years old),was measured to be 395±9 μm (mean±SEM, n=65).The receptor chamber was filled with a 0.01 M PBS solution containing 0.5% HPβCD. Next, the patches were brought on the epidermal surface of the skin. To ensure optimal contact between the patch and the skin, parafilm (American National CanTM,Chicago,USA)was spanned over the patch.Subsequently, the dose chamber was placed on top of the parafilm and the whole set-up was held together with a clamp.Franz diffusion cell samples of the receptor fluid(200 μL)were drawn at regular time intervals from the sample port (1, 2, 5,8,12,20 and 24 h)and the receptor chamber was immediately replenished with 200 μL fresh solution.The spilanthol content in the samples was assayed using the optimized high-throughput fused-core HPLC-UV method (100 μL injection volume and 0.1% FA (v/v) in 70/30 MeOH/H2O (v/v) mobile phase).At the end of the experiment (i.e., after 24 h), the mass balance was constructed and the overall percentage spilanthol recovered was 100±3%(mean±SEM, n=33).The skin permeation parameters were calculated from the plot of cumulative amount of spilanthol permeated through the skin as a function of time [12,13].
3. Results and discussion
For profiling purposes of N-alkylamides,fully porous columns are traditionally used[12].Moreover,these columns have also been applied in high-throughput systems for the isobutylamide spilanthol [13]. However, compared to fused-core based highthroughput systems,the total run time of conventional column based systems is more than doubled, making this new coreshell technology very interesting when analyzing large sample amounts.
Besides the commonly used C18 fused-core columns, where separation efficiency under different conditions was investigated for a wide variety of chemical components [11,15-20],other fused-core columns such as C8, HILIC, RP-amide,peptide, phenyl-hexyl are becoming available. In contrast to C18 fused-core columns, little investigation has been carried out on the column performance of these other stationary phases[21-23].Therefore,we selected the newly available RPamide stationary phase, for which at this time no quality parameters have been investigated.
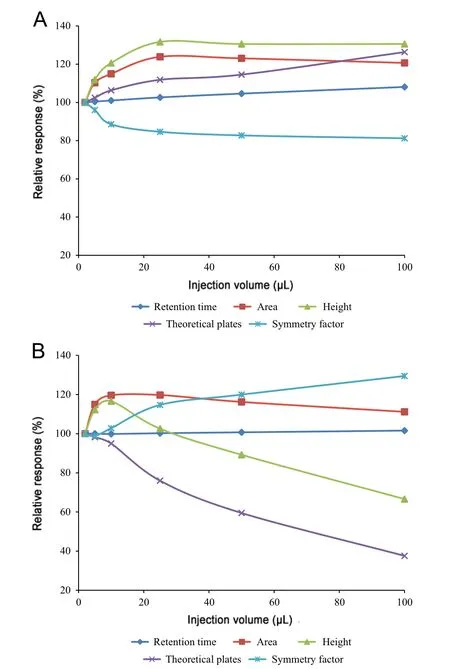
Fig.1 Chromatographic characteristics of PBS (A) and methanol-based (B) samples expressed as percentage relatively to the injection volume of 2 μL(=100%).Conditions:0.1%formic acid in MeOH/H2O (70/30, v/v) mobile phase composition on aged column.
The effect of the injection volume on the performance of RP-amide fused-core HPLC chromatography has been established and demonstrated to be more pronounced than on conventional columns. Varying the injection volume within the 2-100 μL range results in altered performance characteristics. Detailed information about the chromatographic characteristics are presented in Supplementary Table 1,and results were depicted, relative to the 2 μL injection volume, in Fig.1.Moreover, typical chromatograms are shown in Fig.2. The ECV, which is negligible in conventional chromatography,causes significant ECD for fast fused-core columns. The ECD is generally more pronounced with increasing injection volume. Comparing Fig.1A (PBS sample solvent) with 1B(MeOH/H2O, 70/30, v/v sample solvent), it is clear that the influence of the injection volume is confounded with the sample solvent. Using the MeOH based sample solvent(Fig.1B), a linear loss of peak performance is observed in the higher injection volume range (25-100 μL) with a decrease in theoretical plates(N)up to 60%and an increase in symmetry factor(As)with 30%(2-100 μL).This collapse in efficiency was not seen when using PBS as sample solvent. Between 25 and 100 μL injection volume, the chromatographic characteristics remain constant.This can be explained by the relatively weaker solvent strength of PBS, resulting in on-column band compression, whereby solute molecules concentrate at the head of the column [24]. By increasing injection volumes from 2 to 25-100 μL,chromatographic parameters favorably alter,yielding a decrease of 20% in the symmetry factor and an increase of the theoretical plates with 30%. A striking observation is the effect at very low injection volumes,which was not further elaborated at this stage. However, all these observations clearly indicate that the injection volume is a critical quality parameter (i.e. a critical quality attribute (CQA)) for qualitative as well as quantitative fused-core based chromatography.
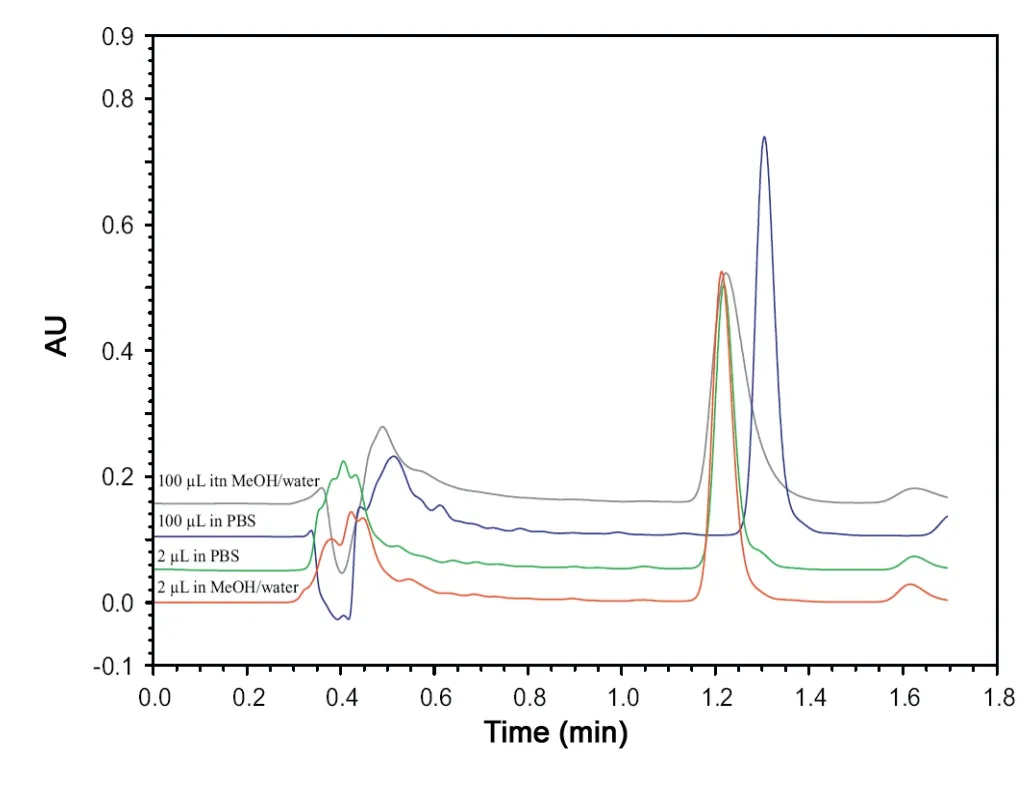
Fig.2 Typical chromatograms demonstrating the effect of injection volume for PBS and MeOH/H2O (70/30, v/v) as sample solvent.Conditions:0.1%formic acid in MeOH/H2O(70/30,v/v)mobile phase composition on aged column.
On the contrary, other investigated factors have a limited contribution to the chromatographic characteristics: no major effects between the investigated mobile phases have been observed and the column age minimally affects the performance, demonstrating the ruggedness and stability of those systems.
Higher injection volumes inherently yield a higher sensitivity.To cope with low concentrated samples generated from e.g.biomedical assays like Franz diffusion cells, 100 μL aqueous samples are preferably introduced on the HALO?RP-amide column. At this high injection volume, the LOD for spilanthol decreased with a factor of 35, from 5.95 μg/mL (for 2 μL injection volume) to 0.17 μg/mL (for 100 μL injection volume).The analytical verification of this high-throughput LC-UV method assured linearity (e.g., R2=0.999) in a working range of 0.57 μg/mL (LOQ) up to 10 μg/mL, with a repeatability of 1.21% (n=3).
Using the FDC flux curves(see Supplementary Fig.S1),the transdermal parameters could be calculated (see Table 1).Neither the plasticizer percentage TEC nor the presence of OA penetration enhancer had an influence on the skin permeation of spilanthol. The spilanthol concentration in the skin (epidermis plus dermis) ranged between 9.815×103ng/mL and 1.889×105ng/mL. At these concentrations local immune effects are certainly to be expected in the skin [15]. Moreover,systemic effects after dermal application are plausible: the steady-state plasma concentration after a 24 h dermal application of a 20% TEC/ 0% OA patch with a surface of 10 cm2was calculated to be 4.58×10-2ng/mL. This is a functional plasma level, resulting in immune-modulatory effects [15].
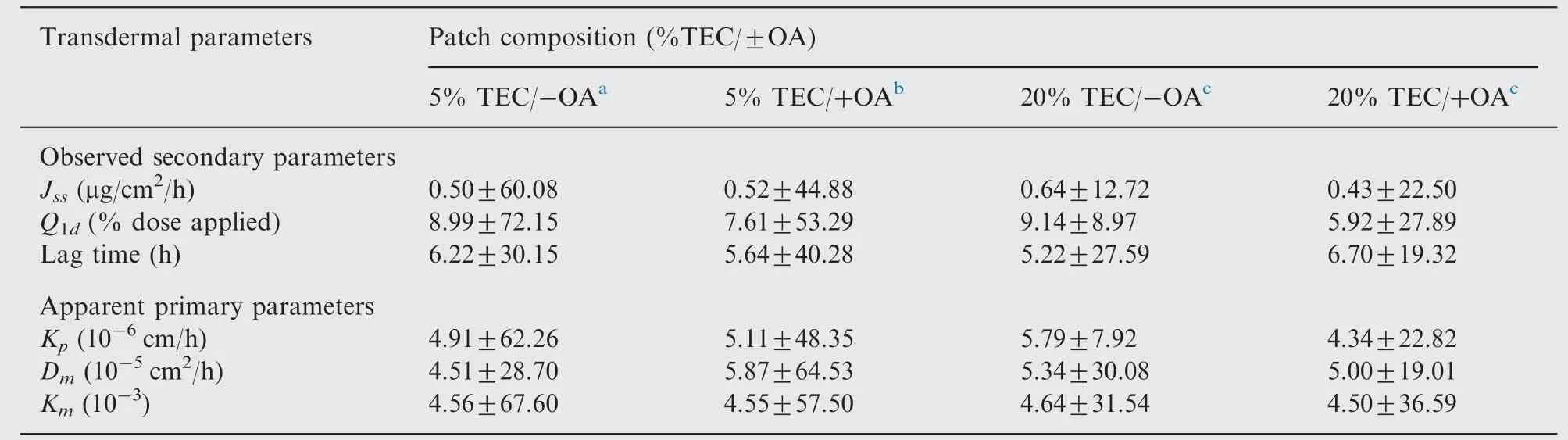
Table 1 Mean transdermal parameters for spilanthol in four different patches [mean±RSD (%)].
4. Conclusions
In contrast to the classic, fully porous HPLC columns, extracolumn parameters demand special attention when fused-core columns are used with conventional HPLC equipment.Not only do the instrument configuration settings contribute to the peak performance, but also the injection volume is more critical than generally thought.Qualitative and quantitative chromatographic characteristics can vary significantly as a function of the injection volume, which in turn can be confounded with the sample solvent. Both the injection volume, the sample solvent and the instrument configuration settings are thus analytical CQAs which should be well defined in method development and validation,and not be overlooked in method transfer. On the contrary, the mobile phase composition and column age do not have a significant influence on the chromatographic characteristics in our operational conditions.
This research is funded by the ''Institute for the Promotion of Innovation through Science and Technology in Flanders(IWT-Vlaanderen)'' to Jente Boonen (No. 091257) and to Matthias D'Hondt(No.101529).We are grateful to Advanced Material Technology (via Achrom (Zulte, Belgium)) for the supply of the HALO?column. Moreover, we acknowledge Bioforce AG and Robertet for providing Spilanthes extracts.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jpha.2013.02.002.
[1] A.J. Alexander, T.J. Waeghe, K.W. Himes, et al., Modifying conventional high-performance liquid chromatography systems to achieve fast separations with fused-core columns:a case study,J. Chromatogr. A 1218 (2011) 5456-5469.
[2] C. Barton, R.G. Kay, W. Gentzer, et al., Development of highthroughput chemical extraction techniques and quantitative HPLC-MS/MS (SRM) assays for clinically relevant plasma proteins, J. Proteome Res. 9 (2010) 333-340.
[3] T. Michel, E. Destandau, C. Elfakir, On-line hyphenation of centrifugal partition chromatography and high pressure liquid chromatography for the fractionation of flavonoids from Hippophae rhamnoides L. berries, J. Chromatogr. A 1218 (2010)6173-6178.
[4] P. Cesla, T. Hajek, P. Jandera, Optimization of two-dimensional gradient liquid chromatography separations, J. Chromatogr.A 1216 (2009) 3443-3457.
[5] A.J. Alexander, L.J. Ma, Comprehensive two-dimensional liquid chromatography separations of pharmaceutical samples using dual fused-core columns in the 2nd dimension, J. Chromatogr.A 1216 (2009) 1338-1345.
[6] W. Song, D. Pabbisetty, E.A. Groeber, et al., Comparison of fused-core and conventional particle size columns by LC-MS/MS and UV: application to pharmacokinetic study, J. Pharm.Biomed. Anal. 50 (2009) 491-500.
[7] J.J. Zheng, D. Patel, Q.L. Tang, et al., Comparison study of porous, fused-core, and monolithic silica-based C-18 HPLC columns for Celestoderm-V ointment (R) analysis, J. Pharm.Biomed. Anal. 50 (2009) 815-822.
[8] J.J.Salisbury,Fused-core particles:a practical alternative to sub-2 μm particles, J. Chromatogr. Sci. 46 (2008) 883-886.
[9] A.Abrahim,M.Al-Sayah,P.Skrdla,et al.,Practical comparison of 2.7 μm fused-core silica particles and porous sub-2 μm particles for fast separations in pharmaceutical process development,J. Pharm. Biomed. Anal. 51 (2010) 131-137.
[10] R.W. Brice, X. Zhang, L.A. Colon, Fused-core, sub-2 μm packings, and monolithic HPLC columns: a comparative evaluation,J. Sep. Sci. 32 (2009) 2723-2731.
[11] P.L. Yang, G.R. Litwinski, M. Pursch, et al., Separation of natural product using columns packed with fused-core particles,J. Sep. Sci. 32 (2009) 1816-1822.
[12] J. Boonen, B. Baert, C. Burvenich, et al., LC-MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol, J. Pharm. Biomed.Anal. 53 (2010) 243-249.
[13] J. Boonen, B. Baert, N. Roche, et al., Transdermal behaviour of the N-alkylamide spilanthol (affinin) from Spilanthes acmella(compositae) extracts, J. Ethnopharmacol. 127 (2010) 77-84.
[15] Y.Hsieh,C.J.G.Duncan,J.M.Brisson,Fused-core silica column high-performance liquid chromatography/tandem mass spectrometric determination of rimonabant in mouse plasma, Anal.Chem. 79 (2007) 5668-5673.
[16] M.C. Pietrogrande, F. Dondi, A. Ciogli, et al., Characterization of new types of stationary phases for fast and ultra-fast liquid chromatography by signal processing based on autocovariance function: a case study of application to Passiflora incarnata L.extract separations, J. Chromatogr. A 1217 (2010) 4355-4364.
[17] S.W. Wang,J. Wen,L.J. Cui, et al., Optimization and validation of a high performance liquid chromatography method for rapid determination of sinafloxacin, a novel fluoroquinolone in rat plasma using a fused-core C-18-silica column,J. Pharm.Biomed.Anal. 51 (2010) 889-893.
[18] F.Gritti,I.Leonardis,J.Abia,et al.,Physical properties and structure of fine core-shell particles used as packing materials for chromatography relationships between particle characteristics and column performance, J. Chromatogr. A 1217 (2010) 3819-3843.
[19] N. Manchon, M. D'Arrigo, A. Garcia-Lafuente, et al., Fast analysis of isoflavones by high-performance liquid chromatography using a column packed with fused-core particles, Talanta 82(2010) 1986-1994.
[20] M. D'Hondt, E. Vangheluwe, S. Van Dorpe, et al., Stability of extremporaneously prepared cytarabine, methotrexate sodium,and methylprednisolone sodium succinate, Am. J. Health Syst.Pharm. 69 (2012) 232-240.
[21] D.V. McCalley, Evaluation of the properties of a superficially porous silica stationary phase in hydrophilic interaction chromatography, J. Chromatogr. A 1193 (2008) 85-91.
[22] W.K.Way,W.Campbell,Ascentis?Express RP-Amide Expands the Selectivity of Fused-Core Particle Technology HPLC Columns, LC-GC North America The Application Notebook 1,Supelco/Sigma-Aldrich, 595 North Harrison Road, Bellefonte,PA 16823, 2008.
[23] M. D'Hondt, B. Gevaert, S. Stalmans, et al., Reversed-phase fused-core HPLC modeling of peptides, J. Pharm. Anal 3 (2)(2013) 93-101.
[24] J.M.Miller,in:Chromatography Concepts and Contrasts,second ed., John Wiley & Sons Inc., New Jersey, 2005.
 Journal of Pharmaceutical Analysis2013年5期
Journal of Pharmaceutical Analysis2013年5期
- Journal of Pharmaceutical Analysis的其它文章
- A novel and rapid microbiological assay for ciprofloxacin hydrochloride
- Determination of sodium hyaluronate in pharmaceutical formulations by HPLC-UV
- Copper interactions with DNA of chromatin and its role in neurodegenerative disorders
- Application of LC-MS/MS for quantitative analysis of glucocorticoids and stimulants in biological fluids
- In situ modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in its formulation
- Kinetic performance comparison of fully and superficially porous particles with sizes ranging between 2.7 μm and 5 μm: Intrinsic evaluation and application to a pharmaceutical test compound
