A novel t(3;12)(q21;p13) translocation in a patient with accelerated chronic myeloid leukemia after imatinib and nilotinib therapy
Ayda Bennour, Ikram Tabka, Yosra Ben Youssef, Zahra Kmeira, Abderrahim Khelif, Ali Saad, Halima Sennana
1Department of Cytogenetics, Molecular Genetics and Reproductive Biology, Farhat Hached University Teaching Hospital, Sousse 4000, Tunisia; 2Department of Clinical Hematology, Farhat Hached Hospital, Sousse 4000, Tunisia
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder of the pluripotent hematopoietic progenitor cells and is characterized by massive proliferation and accumulation of myeloid cells that differentiate normally1,2.The cytogenetic hallmark of CML is the Philadelphia (Ph) chromosome that results from a reciprocal t(9;22)(q34;q11.2) translocation or its variants t(V;9;22)3.The molecular consequence of the t(9;22)(q34;q11.2) translocation is the formation of the BCR/ABL fusion gene, which is usually located on the Ph chromosome4.Clinically, CML progresses through three distinct phases:the easily controlled chronic phase, the ill-defined unstable accelerated phase, and the terminal blastic phase5.
The latter phase resembles acute leukemia and is highly refractory to chemotherapy with ≤20% response rate and a median survival period of 3 months to 6 months5,6.Majority of the CML patients showed changes only in the Ph chromosome throughout the chronic phase.As CML progresses to the advanced phases, the Ph chromosome-positive (Ph+) cells acquire new karyotypic abnormalities, most often a second copy of the Ph chromosome, i(17q), and trisomy 8 and 19.The increased genetic instability of the Ph+ leukemic clone facilitates the emergence of subclones with highly malignant phenotypes6.In this paper, we report a patient with Ph+ CML who acquired a novel additional chromosomal aberration t(3;12)(q21;p13)during the course of disease progression.This aberration was con firmed with fluorescence in situ hybridization (FISH).
Case report
In October 2005, a 28-year-old man was referred to our hospital because of leukocytosis, splenomegaly, and weight loss.Peripheral blood count showed hemoglobin: 9.4 g/dL;platelets: 253×109/L; white blood cells (WBC): 292×109/L with 32% neutrophils, 2% lymphocytes, 3% eosinophils, 3%basophils, 58% immature granulocytes, and 2% blasts.Myeloid hyperplasia of the bone marrow (BM) with high proportion of cells at different stages of maturation, moderate eosinophilia without an excess of blasts, and 3% blasts was found.Cytogenetic analyses were performed on BM cells.Metaphases were obtained after short-term unstimulated cultures (24 and 48 h).The chromosomes were identified by heating R-bands through the Giemsa (RHG) technique.The chromosomes were classified following the Guidelines for the International System for Human Nomenclature7.FISH was performed by using whole-chromosome paint probes for chromosomes 3 (spectrum green)/12 (spectrum orange) (Vysis, Inc., Downers Grove, IL, USA).All of the details concerning the pretreatment of slides and hybridization were carried out according to the instructions accompanying the probes.The chromosomes were counterstained with 4’,6-diamidino-2-phenylindole and viewed with an Axioskop fluorescence microscope (Zeiss, Germany)by using triple excitation/emission bandpass filters.At least 10 metaphase cells were analyzed.
Chromosomal analysis revealed 46,XY, t(9;22)(q34;q11).The patient was diagnosed as having CML in the chronic phase.Treatment was initiated with hydroxyurea (1,500 mg daily dose).Eight months after the treatment, the WBC of the patient decreased to 4.7×109/L (with 43% neutrophils), platelets:132×109/L; hemoglobin: 14 g/dL.The patient was then treated with imatinib mesylate (400 mg daily dose).After 6 months,cytogenetic analysis revealed a major cytogenetic response with 10% Ph+ cells.Twelve months after imatinib treatment initiation,a repeat R-banding karyotype showed a complete cytogenetic response (CCyR).After 24 months, the patient’s blood count showed WBC: 5.5×109/L (65% neutrophils, 33% lymphocytes,1% monocytes, and 1% basophils); hemoglobin: 13.4 g/dL;platelets: 177×109/L.Karyotype analysis showed additional chromosomal abnormality (trisomy 8): 47,XY,+8,t(9;22)(q34;q11)/46,XY.Subsequently, the dose of imatinib was raised to 600 mg daily.After 6 months, CCyR was reached again.
After 24 months, the patient’s blood count showed WBC:5.3×109/L (58% neutrophils, 36% lymphocytes, 5% monocytes,and 0.5% eosinophils); hemoglobin: 11.5 g/dL; platelets:162×109/L.Cytogenetic analysis revealed two cell clones with additional chromosomal abnormalities: 47, XY, t(9;22)(q34;q11),+der(22)t(9;22)/48, idem, +8/46, XY.
Treatment with nilotinib (200 mg daily) was initiated, and 2 months later, the blood count showed WBC: 6.7×109/L(66% neutrophils, 26% lymphocytes, 5% monocytes, and 0.5%eosinophils); hemoglobin: 14.2 g/dL; platelets: 219×109/L.
CCyR and complete molecular response were reached throughout 3 months until the patient discontinued nilotinib treatment.Hematologic parameters showed that hemoglobin was 13.8 g/dL, platelets were 273×109/L, and WBC was 60.3×109/L (61% neutrophils, 7% lymphocytes, 4% monocytes,1% erythroblasts, 20% immature granulocytes, and 7% blastic cells) (Table 1).
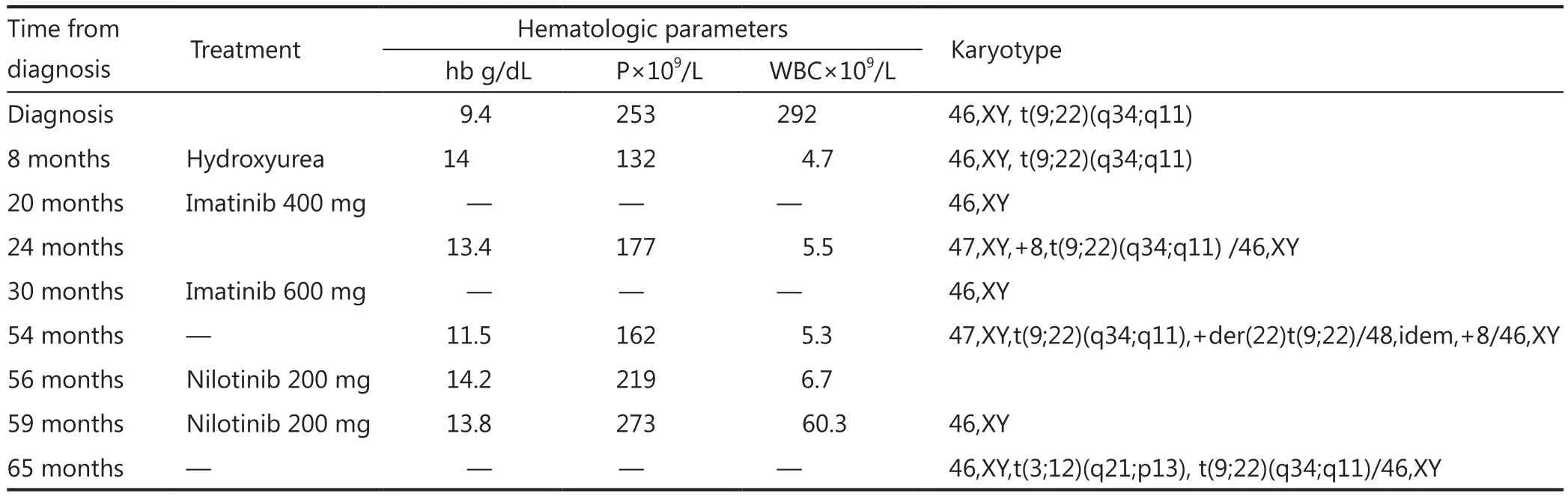
Table 1 Clinical data of patient
Bone marrow aspiration concluded to an accelerated phase with 14% blastic cells (17% promyelocytes, 34% neutrophils,20% eosinophils, and 6% erythroblasts) as well as an average bone marrow cellularity with rare megakaryocytes, platelets, and 21% myelocytes and metamyelocytes.Conventional karyotype analysis revealed additional recurrent translocation with t(9;22):46,XY,t(3;12)(q21;p13), t(9;22)(q34;q11) (Figure 1),which was confirmed with FISH by using whole-chromosome paint probes for chromosomes 3 and 12 (Figure 2).
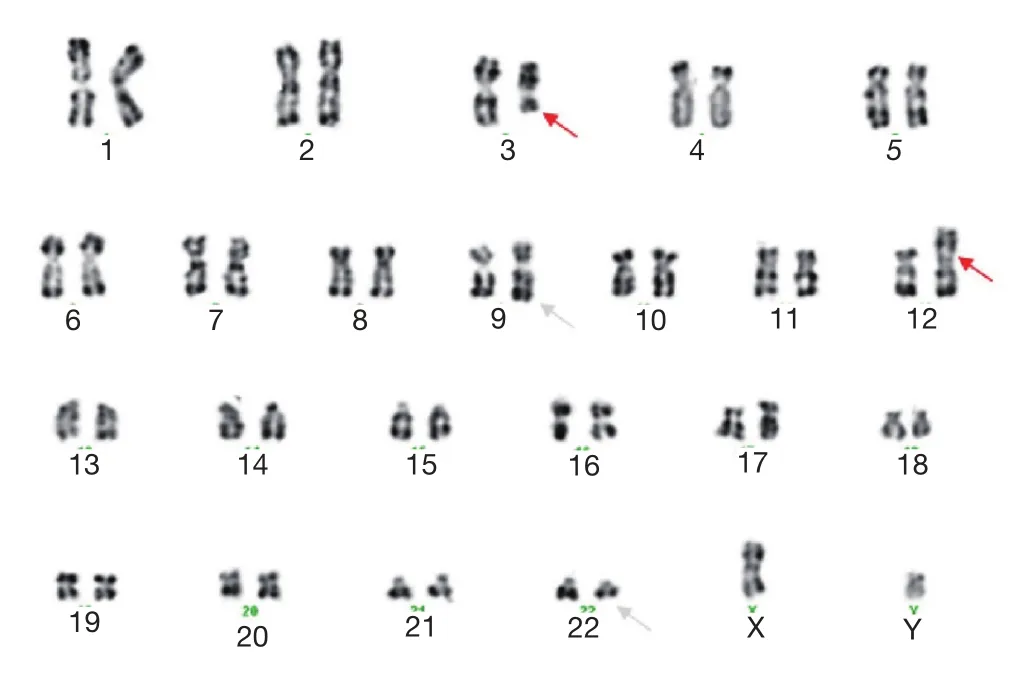
Figure 1 Karyotype from metaphase cell of the patient showing 46,XY,t(3:12)(q21;p13), t(9;22)(q34;q11).Chromosomes were identified by using RHG banding.The arrowheads indicate the location of the breakpoint on derivative chromosomes.
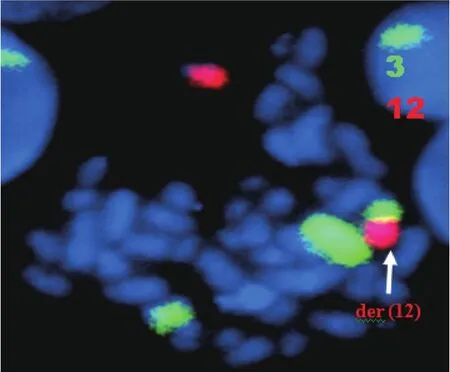
Figure 2 FISH analysis of the metaphase cell of the patient:chromosome painting [chromosomes 3 (green) and 12 (red)]displays an insertion of chromosome 3 material into chromosome 12.
Discussion
We report a cytogenetically identical t(3;12)(q21;p13)translocation in one patient with accelerated CML.To our knowledge, the t(3;12)(q21;p13) translocation has not yet been described in the literature in patients with CML8.However,two cases of refractory anemia with t(3;12)(q21;p13) have been reported9,10.
All attempts to induce remission were unsuccessful, which suggests that this chromosomal abnormality may be associated with poor prognosis.
Structural abnormalities affecting the long arm of chromosome 3, mostly including paracentric inversions inv(3)(q21q26), insertions ins(3)(q2lq25q26), homologous translocations t(3;3)(q2;q26), and translocations between 3q26,and other chromosomes such as t(3;21)(q26;q22) and t(2;3)(q21;q26)(5;7;10), have been described in 140 cases11-13.These specific chromosomal rearrangements have been demonstrated in all French–American–British subtypes of acute myeloid leukemia except M3.These chromosomal rearrangements have also been shown in rare cases of myelodysplastic syndrome(MDS), and in the blastic crisis of CML (CML-BC)14.
The t(3;12)(q26;p13) translocation has recently been reported as a new recurrent translocation in MDS and CMLBC15.This translocation involves the ETV6 gene (formerly named TEL), a new member of the ETS gene family of transcription factors at 12p13 and the EVI1 (zinc finger transcription factor) gene at 3q26.This translocation, which also generates the TEL/EVI1 fusion gene, could lead to the inappropriate expression of EVI1 protein driven by the TEL promoter and could contribute to malignant transformation15.The t(3;12) translocation involving 3q21 breakpoint has never been reported in CML until that time8.
The incidence of 3q anomalies with breakpoints in q21 and q26 is shown to be higher than previously estimated.These anomalies can be considered as frequent and specific chromosomal changes in myeloid malignancies11,16.The breakpoints in both 3q regions are scattered over considerable distances17,18.
Thus, similar to the t(3;12)(q21;pl3) translocation, the t(3;12)(q26;pl3) translocation appears as a rare but nonrandom event observed in various myeloid leukemia subtypes, which are frequently associated with dysplasia of megakaryocytes,multilineage involvement, short duration of any blastic phase,and extremely poor prognosis.
In contrast to 3q26, the precise role of the sequences in 3q21 is uncommon and less well described.The breakpoints are clustered, and the 10 breakpoints that have been mapped are located within a segment no more than 30 kb in size19-21.However, no candidate genes for leukemia have been described.
The most intriguing question generated by this study is whether the formation of t(3;12)(q21;p13) in the t(9;22)(q34;q11) positive clone is related to therapy or to the accelerated phase of CML.
The co-existence of t(9;22) and t(3;26) has been reported in only one patient with CML22.The t(3;12)(q26;p13)translocation emerged as a sole additional chromosomal abnormality 16 months prior to the blastic crisis.The patient received combination chemotherapy with cytosine arabinoside and daunorubicin.The patient achieved complete remission after bone marrow transplantation22.
The patient received tyrosine kinase inhibitor (TKI) therapy for a total of 4 years.However, this dose of TKI therapy is most likely related to the development of t(3;12).
Therefore, we can speculate that the acquisition of trisomy 8 and the additional Ph chromosome followed by the acquisition of t(3;12) in this case is either a result of the natural history of the CML characterized initially by t(9;22) and +8 or re flects the simultaneous development of two clonal myeloid populations.
The puzzling but intriguing role of trisomy 8 in the disease progression in these patients remains uncertain.The trisomy 8 in this case may represent either the t(3;12) clone or the Ph+ clone that developed simultaneously.
The hypothesis may not be consistent with the current dogma; 100% of BCR-ABL1 + cells bear concomitant trisomy 8 cells, which is thought to be associated with an accelerated phase although blast cells were not found and the patient has a WBC of 7.2×109/L.
We conclude that some patients with CML are either in the accelerated phase with coexistence of t(9;22) and t(3;12)with or without trisomy 8 or would advance to blast crisis and simultaneously carry BCR-ABL and duplicate Ph, trisomy 8, or other cytogenetic abnormalities, such as the t(3;12) detected in this case.This progression may occur after hydroxyurea or TKI therapy.
Conclusions
We have described a patient with a novel unbalanced t(3;21)(q26;q22) translocation emerging with t(9;22) in the accelerated phase of CML.To our knowledge, this additional chromosomal aberration that involves breakpoint at 3q21 has not yet been described.This aberration is thought to play a crucial role in CML progression.
Results show that additional cytogenetic abnormalities are frequently detected and are significantly associated with poor overall survival in patients with Ph+ CML after failure of imatinib treatment.This finding indicates that conventional cytogenetics on metaphases remain mandatory at diagnosis and during follow-up and should not be replaced by techniques that analyze only BCR-ABL.
The decreased efficacy of nilotinib in the context of additional cytogenetic abnormalities may re flect intrinsic aggressiveness of the disease, thus prompting the close monitoring of this disease.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Deininger MW, Goldman JM.Chronic myeloid leukemia.Curr Opin Hematol 1998;5:302-308.
2.Sawyers CL.Chronic myeloid leukemia.N Engl J Med 1999;340:1330-1340.
3.Rowley J.A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining.Nature 1973;43:290-293.
4.Deininger MW, Goldman J, Melo J.The molecular biology of chronic myeloid leukemia.Blood 2000;96:3343-3356.
5.Sacchi S, Kantarjian H, O’Brian S, Cortes J, Rios MB, Giles FJ, et al.Chronic myelogenous leukemia in nonlymphoid blastic phase:analysis of the results offirst salvage therapy with three different treatment approaches for 162 patients.Cancer 1999;86:2632-2641.
6.Bernstein R.Cytogenetics of chronic myelogenous leukemia.Semin Hematol 1988;25:20-34.
7.ISCN (2009): An international system for human cytogenetic nomenclature.In: ShaVer LG, Tommerup N (eds) Karger.
8.Mitelman F, Johansson B, Mertens F(Eds.) Mitelman Database of Chromosome Aberrations in Cancer (2012).http://cgap.nci.nih.gov/Chromosomes/Mitelman
9.Fleischman EW, Reshmi S, Sokova OI, Kirichenko OP,Konstantinova LN, Kulagina OE, et al.Increased karyotype precision using fluorescence in situ hybridization and spectral karyotyping in patients with myeloid malignancies.Cancer Genet Cytogenet 1999;108:166-170.
10.Mauritzson N, Johansson B, Rylander L, Albin M, Str?mberg U,Billstr?m R, et al.The prognostic impact of karyotypic subgroups in myelodysplastic syndromes is strongly modi fied by sex.Br J Haematol 2001;113:347-356.
11.Fonatsch C, Gudat H, Lengfelder E, Wandt H, Silling-Engelhardt G, Ludwig WD, et al.Correlation of cytogenetic findings with clinical features in 18 patients with inv(3)(q21q26) or t(3;3)(q21;q26).Leukemia 1994;8:1318-1326.
12.Horsman DE, Gascoyne RD, Barnett M.Acute leukemia with structural rearrangements of chromosome 3.Leukemia Lymphoma 1995;16:369-377.
13.Secker-Walker LM, Mehtia A, Bain B.Abnormalities of 3q21 and 3q26 in myeloid malignancy: a United Kingdom Cancer Cytogenetic Group study.Br J Haematol 1995;91:490-501.
14.Heim S, Mitelman F.Cancer Cytogenetics.New York: Alan R.Liss,Inc, 1987:65-128.
15.Raynaud SD, Baens M, Grosgeorge J, Rodgers K, Reid CD, Dainton M, et al.Fluorescence in situ hybridization analysis of t(3;12)(q26;p13): a recurring chromosomal abnormality involving the TEL gene (ETV6) in myelodysplastic syndromes.Blood 1996;88:682-689.
16.Bellomo MJ, Parlier V, Muhlematter D, Grob JP, Beris P.Three new cases of chromosome 3 rearrangements in band q21 and q26 with abnormal thrombopoiesis bring further evidence to the existence of a 3q213q26 syndrome.Cancer Genet Cytogenet 1992;59:138.
17.Morishita K, Parganas E, Willman CL, Whittaker MH, Drabkin H,Oval J, et al.Activation of the EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300–400 kilobases on chromosome 3q26.Proc Natl Acad Sci 1992;89:3937-3941.
18.Levy ER, Parganas E, Morishita K, Fichelson S, James L, Oscier D,et al.DNA rearrangements proximal to the EVI1 locus associated with the 3q21q26 syndrome.Blood 1994;83:1348-1354.
19.Suzukawa K, Parganas E, Gajjar A, Abe T, Takahashi S, Tani K, et al.Identification of a breakpoint cluster region 3’ of the Ribophorin I gene at 3q21 associated with the transcriptional activation of the EVIl gene in acute myelogenous leukemia with inv(3)(q21q26).Blood 1994;84:2681-2688.
20.Pekarsky Y, Zabarovsky E, Kashuba V, Drabkin H, Sandberg AA, Morgan R, et al.Cloning of breakpoints in 3q21 associated with hematologic malignancy.Cancer Genet Cytogenet 1995;80:1-8.
21.Rynditch A, Pekarsky Y, Schnittger S, Gardiner K.Leukemia breakpoint region in 3q21 is gene rich.Gene1997;193:49-57.
22.Nakamura Y, Nakazato H, Sato Y, Furusawa S, Mitani K.Expression of the TEL/EVI1 fusion transcript in a patient with chronic myelogenous leukemia with t(3;12)(q26;p13).Am J Hematol 2002;69:80-82.
 Cancer Biology & Medicine2013年1期
Cancer Biology & Medicine2013年1期
- Cancer Biology & Medicine的其它文章
- Combined transfection of Bcl-2 siRNA and miR-15a oligonucleotides enhanced methotrexate-induced apoptosis in Raji cells
- Guidelines for the diagnosis and treatment of follicular lymphoma in China
- Reduced-intensity conditioning allogeneic stem cell transplantation in malignant lymphoma: current status
- Adenoid cystic carcinoma in the maxillary gingiva: a case report and immunohistochemical study
- Spontaneous pineal apoplexy in a pineal parenchymal tumor of intermediate differentiation
- Research development of the relationship between thymidine phosphorylase expression and colorectal carcinoma
