Effects of Zn2+ and Cu2+ on loach ovaries and ova development
Jian-Xun TANG, Jun-Rong LI, Zhong-Liang LIU, Hua ZHAO, Xiao-Min TAO, Zhang-Shun CHENG
1. College of Agriculture and Bio-engineering, Jinhua Polytechnic Institute, Jinhua, 321007, China
2. College of Pharmacy and Material Engineering, Jinhua Polytechnic Institute, Jinhua, 321007, China
The problem of heavy metal pollution in aquatic environments has become an increasingly serious concern over the past decades in China (Wang et al,2008). The resulting pollution that accompanies industrialization has severely challenged the survival of aquatic animals due to strong toxicity and the bioaccumulation of heavy metals. Of these pollutants,metal ions accumulate not only in the skin, muscles, liver,and kidneys (Al-Weher S et al, 2008; Dutta T et al, 2001;Has S et al, 2008; Migliarini et al, 2005), but also in the gonads, which poses a serious threat to the reproductive success of many aquatic species and by extension the overall population of those animals (Abou El-Naga et al,2005; da Cruz et al, 2007; Tang et al, 2010). Accordingly,to maintain biodiversity and protect the breeding system of aquatic fauna, it is necessary to conduct studies on the accumulation of heavy metals in a fish’s reproductive organs and the resulting stress on their ova to thereby establish correlations between metal accumulation and ion concentration.
Zn2+and Cu2+are commonly found in bodies of water polluted by heavy metals. Neither biodegrades easily and these metals are amplified via bioaccumulation as they pass through food chain. To explore how heavy metal accumulation strains the development of the ovaries and ova, in this study we used Loaches (Misgurnus anguillicaudatus), a fish species known to tolerate pollution well (Gao et al, 2003; Wang et al, 2003) as a model. By doing so, we1hope to find results that will prove useful in gauging the impact assessment of aquatic organisms, diagnostics of environmental pollution, and biodiversity conservation.
MATERIALS AND METHODS
Animal specimens and reagent materials
Two year old loaches (n=220) were purchased from a local farmer’s market for use as a testing species. The overall mean length of the fish was 13.2±3.5 cm and the mean body weight 37±0.48 g.
Concentrated solutions (1 000 mg/L) of ZnSO4(AR)and CuCl2(GR) were first prepared before being diluted into the corresponding concentrations as needed throughout the course of the experiment. HNO3(AR) and HCIO4(AR) were mixed at a ratio of 4:1 before use.
Instruments and equipments
The following equipment were used over the duration of this study: an H-6800 Aerating Pump (China); an IRIS Intrepid ER/S-model ICP atomic emission spectrometer (Thermo Elemental Co., USA); a CKX41 inverted fluorescent microscope (HQ2592×1944)(Olympus Co., Japan); an LSP far-infrared cooking stove (China); a KD (freeze) slicing machine (China);and a DGG-9070A drying oven with an electrical thermostatic wind drum (China).
Experimental design
The experiment was carried out indoors. During testing, the water quality parameters were maintained consistently: pH 6.3-6.5, DO 5.1-6.2 mg/L; temperature at 8-12°C (measured daily); average total water hardness at 2.67 mmol/L; and average alkalinity at 2.6 mmol/L.
Polyethylene plastic aquariums (40 cm×30 cm×45 cm)were used as exposure devices, and 20 L of tap water, in which no Zn2+and Cu2+was detected, was added to each tank and then aerated for more than 2 days prior to being used. The loaches accepted for testing were visually inspected to be disease-free, injury-free and active, with an overall mortality rate less than 5% during the testing period. The fish were acclimated to lab conditions for 5 days before the experiment began. Over the course of testing, there was no water exchange in the plastic aquariums and the fish were not fed. An aerating pump was used to keep dissolved oxygen above 5 mg/L.
In total, 220 similarly sized loaches were randomly placed into four testing groups. The control group was placed in water with no heavy metals present, while the other three were given different concentrations of Zn2+and Cu2+similar to actual ion concentrations in the local water bodies based on the Chinese Standard of Water Quality for Fisheries (GB11607-1989): Zn2+concentrations of 1.00, 2.50 and 5.00 mg/L paired with Cu2+concentrations of 0.10, 0.25 and 0.50 mg/L. The fish were held in each aquarium and three parallels for each concentration over the course of the 20-day experiment. Each individual was weighed, boiled, and dried prior to analysis, during which ovary slices from
different groups and at different times (before testing, 5-day, 10-day and 20-day) were cut and observed.
Statistical analysis
The processing method of statistics for the testing results follows the method described by Nan Xu-Yang(Nan, 2009). All data was analyzed using SPSS 11.0 data processing system (SPSS Inc., Chicago, USA) for analysis of variance. The values for individual experiments were collected to calculate the mean value and the standard deviation/error to make comparisons. The statistical significance of the difference between means was determined using one-way ANOVA, with P<0.05 being statistically significant.
RESULTS
Accumulating rule of Zn2+ and Cu2+ in ovaries
Before exposure to the metals, Zn2+and Cu2+, all loaches were tested to ensure that no metals were present in the ovaries. By 10 days of exposure, the concentrations of Zn2+and Cu2+in loach ovaries across all groups being kept in water with varying concentrations of heavy metals had increased. On day 20, the concentration of both ions showed an increasing trend, but between day 10 and day 20 the concentrations of Zn2+and Cu2+in the ovaries seemed to decline significantly when compared to ion concentrations of the groups when they had been exposed for less than 10 days (P<0.05).
The cumulative capacity of Zn2+and Cu2+in the ova was positively related to concentration of the metals in aqueous solution (P<0.05). The accumulation of Zn2+and Cu2+in the ovaries presented as a function of exposure time and dose effects are shown in Table 1. In the control group, no Zn2+and Cu2+were detected.
Table 2 shows the regression equations and correlation coefficients for ovaries, indicating a positive relationship between Zn2+and Cu2+accumulation and exposure time. Relationships between accumulation of Zn2+and Cu2+in the ovaries and exposure time are shown in Figures 1 and 2.
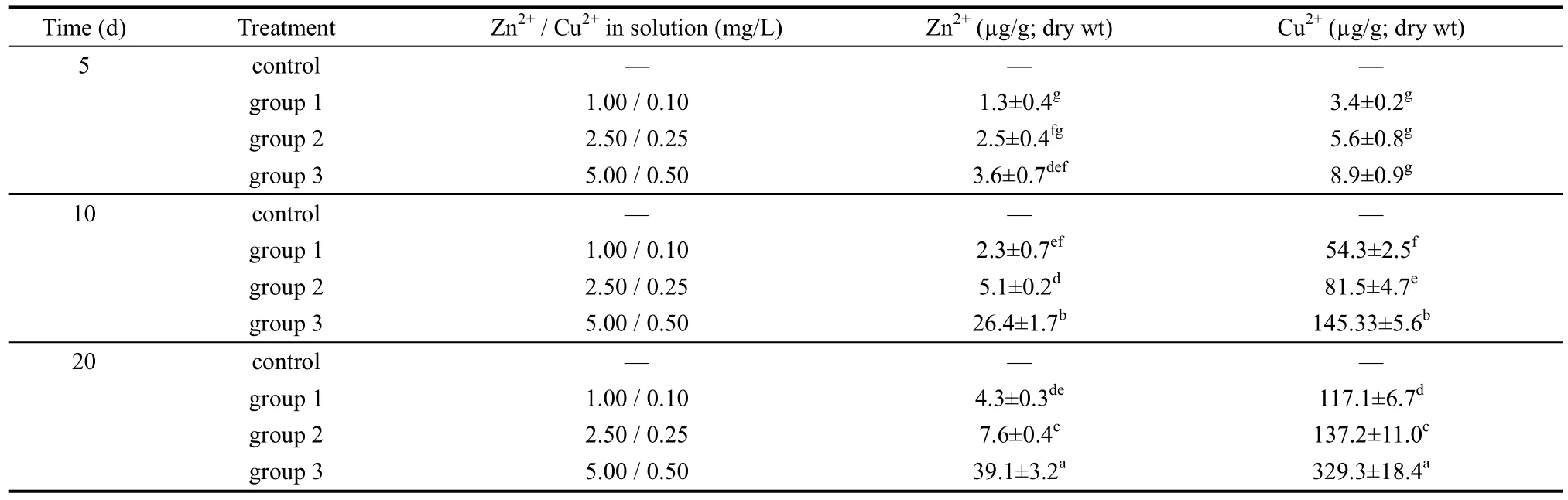
Table 1 Accumulation of Zn2+ and Cu2+ in ovary at different time intervals (n=200)

Table 2 Regression equations and coefficients of accumulation for Zn2+ and Cu2+ with exposure time in ovary
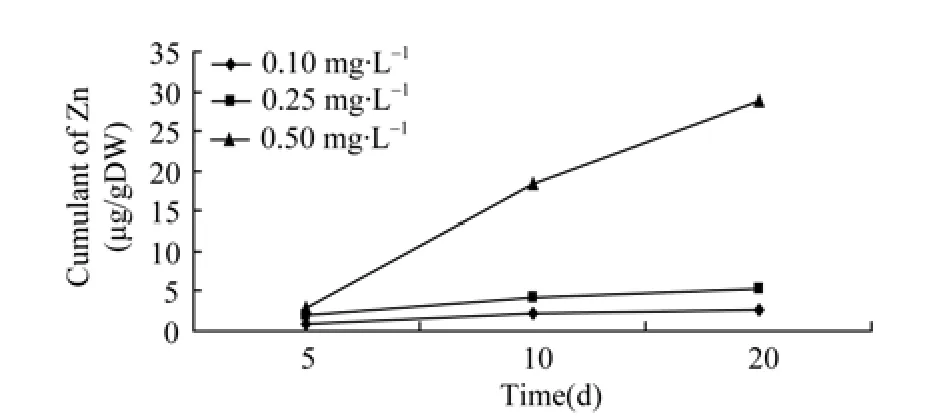
Figure 1 Relationship of accumulation of zinc and time in ovary
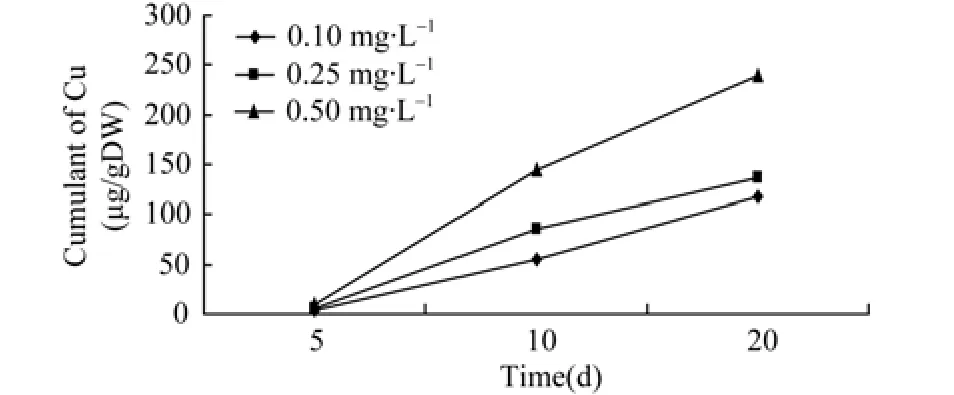
Figure 2 Relationship of accumulation of copper and time in ovary
Stress on ovary development from Zn2+ and Cu2+
The ovaries and ova in the controls, in which the metals were not detected, developed normally. Zn2+and Cu2+accumulated rapidly in the ovaries of all treatment groups exposed to mixtures of Zn2+and Cu2+. The fish showed on the atrophy and cytoplasmic leakage in the ova, suggesting histological damage in the ovaries by day 10 of exposure. By day 20, the ova showed symptoms of severe degeneration, the mutual bonding of cells and severe atrophy (Figure 3).
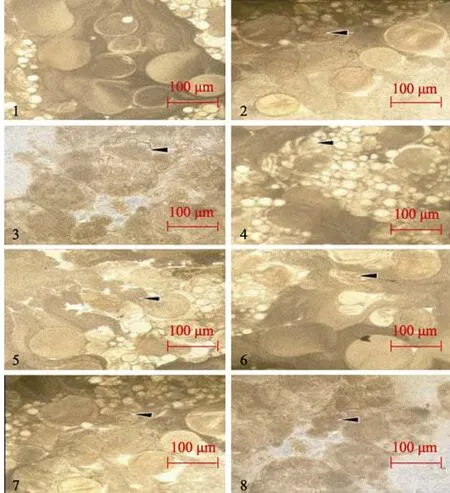
Figure 3 Effects of joint attack of Zn2+ and Cu2+on ovaries and ova
DISCUSSION
As trace elements of an organism, Zn2+and Cu2+are integral parts of cellular structure and enzyme compounds. Zn2+is one of the important growth factors and plays a vital role in growth, development and appetite, etc. for fishes and other aquatic organisms (Al-Weher S, 2008; Ebrahimi M, 2007). However, studies have shown that higher doses and longer exposure are not beneficial, and have toxic effects on aquatic organisms (Zhou et al, 2002). As for Cu2+, it is also a type of nutritive trace element necessary for fish growth(Gao et al, 2003), and it performs important functions for the physiological activities in fishes. The physiological metabolism will be negatively influenced if either Zn2+or Cu2+is lacking, but overdosing brings adverse impacts,especially in the case of Cu2+. In general, the harmfulness of Zn2+is relatively small as compared with Cu2+, which is highly toxic to aquatic organisms in stronger dosages (Wang et al, 2006).
As mentioned above, the amount of Zn2+and Cu2+accumulated in the ovaries was related to the concentration of Zn2+and Cu2+in the aquatic environment and the duration of exposure to the metals.The cumulative amount of heavy metal in the ovaries rises with the concentration of Zn2+and Cu2+to a certain level alongside the exposure period, demonstrating the significant effect of time and doses. However, the accumulation rate tended to decline when exposure time exceeded 10 days. It could be that when organisms are over-exposed to heavy metals such as Zn2+and Cu2+, the metals activate the transcription of metallothionein genes in the organs, resulting in more genes being expressed.Consequently, these expressed genes combine the metals that have entered the cells with those new synthetic proteins (Allen, 1995). Alternatively the cells overexposed to the heavy metals are in a state of “saturation”and thus can no longer absorb more heavy metals. As exposure continues, the level of heavy metal accumulation declined in the ova, which was more obvious in the cases of different concentrations of Zn2+(Figure 1). As for the accumulation of Cu2+under different concentrations, the higher the concentration of Cu2+, the slower the accumulation tended to be. It seemed that the cumulated amount of Cu2+increased in the ova between 10 to 20 days, when the concentration was low, i.e., 0.1 mg/L (Figure 2).
Ultimately, these results indicate that the ability of the ovaries to detoxify decreased and the ova were damaged as a result of 20 day’s exposure to heavy metal at higher concentrations. Long term exposure to heavy metals will likely then further damage the overall function of ovaries (Figure 3), which is in agreement with previous studies regarding organs in aquatic organisms being damaged by heavy metals (Wang et al,2003; Zhang et al, 2009; Zhou et al, 2010).
Our results also showed that when both Zn2+and Cu2+were at a low concentration (1.00 and 0.10 mg/L,respectively), the physiological activities and ovarian development of loaches were not affected by Zn2+and Cu2+within 20 days of exposure, though future studies are needed to determine the effects of long-term exposure at similar concentrations. As the concentration of the metals and/or exposure time increased the function of ovarian development and the metabolism of ova suffered serious damage. Studies of single metal exposure showed that female sword fish (Xiphophorus Helleri) on average laid only 17 eggs when exposed to Cu2+(0.12 mg/L) for 140 days, compared with the 228 eggs laid in the control group (James et al, 2003). But the effect of single metal exposure can be very different from multi-metal exposure on aquatic organisms in terms of toxicity accumulation. In a natural environment, a synergic or antagonistic action may occur as different heavy metals usually co-exist (Zhang et al, 2008). In addition, factors such as water temperature, forms of heavy metals, existence of other chemical ions and changes in other environmental variables may affect the heavy metal accumulation and cause harm to aquatic organisms (Li et al, 2002; Tang et al, 2010). Further studies, therefore, are needed to address these issues.
Abou El-Naga EH, El-Mosehy KM, Hanmed MA. 2005. Toxicity of cadmium and copper and their effect on some biochemical parameters of marine fish mugil sheheli. Egyptian Journal of Aquatic Resources,31(2): 60-71.
Allen P. 1995. Soft-tissue accumulation of lead in the blue Tilapia,Oreochromis aureus (Steindachner) and the modifying effects of cadmium and mercury. Biological Trace Element Research, 50(3): 193-208.
Al-Weher SM. 2008. Levels of heavy metal Cd, Cu and Zn in three fish species collected from the North Jordan Valley, Jordan. Jordan Journal of Biological Sciences, 1(1): 41-46.
da Cruz ACS, Couto BC, Nascimento IA, Pereira SA, Leite MBNL,Bertoletti E, Zagatto P. 2007. Estimation of the critical effect level for pollution prevention baced on oyster embryonic development toxicity test:The search for reliability. Environment International, 33(4): 589-595.
Dutta TK, Kavira JA. 2001. Acute toxicity of cadmium to fish Labeo rohita and copepod Diaptomus forbesi pre-exposed to CaO and KMnO4.Chemospher, 4(8): 955-958.
Ebrahimi M. 2007. Effects of in vivo and in vitro zinc and cadmium treatment on sperm steroidogenesis of the African catfish Clarias gairepinus. Pakistan Journal of Biological Sciences, 10(17): 2862-2867.Gao XL, Qi FS, Luo HY, Wang LM, Li YH. 2003. Acute toxicity and joint toxicity test of Cu, Hg and Cr on Misgurnus anguillicaudatus.Reservoir Fisheries, 23(2): 63-64. (in Chinese)
Has Sch?n E, Bogut I, Kralik G, Bogut S, Horvati? J, Caci? M. 2008.Heavy metal concentration in fish tissues inhabiting waters of Busko Blato reservoir (Bosnia and Herzegovina). Environment Monitoring Assessment, 144(1-3): 15-22.
James R, Sampath K, Edward DS. 2003. Copper toxicity on growth and reproductive potential in an ornamental fish, Xihophorus helleri. Asian Fisheries Science, 16: 317-320.
Li SX, Sun HW, Wang YQ, Dai SG. 2002. Bioconcentration and partition behaviors of tributyltin. Acta Scientiae Circumstantiae, 22(6):726-731. (in Chinese)
Migliarini B, Campisi A M, Maradonna F, Truzzi C, Annibaldi A,Scarponi G, Carnevali O. 2005. Effects of cadmium exposure on testis apoptosis in the marine teleost Gobius niger. General and Comparative Endocrinology, 142(1-2): 241-247
Nan XY. 2009. Toxicity effects of heavy metals Cu, Zn and Cd and influence to gobble up ability of white blood cells in loach.Agricultural Science of Shanxi, 2: 40-43. (in Chinese)
ang JX, Xing CH, Liu ZL, Cheng ZS, Li JR. 2010. Accumulation of heavy metals (Cu and Pb) in the ovary of Misgurnus anguillicaudatus and the subsequent effects on ova development. Oceanologia et Limnologia Sinica, 41(3): 386-390. (in Chinese)
Wang RL, Ma GZ, Fang ZQ. 2006. Safety assessment and acute toxicity of copper, cadmium and zinc to white clound mountain minnow tanichthys albonubes. Fisheries Science, 25(3): 117-120. (in Chinese)
Wang YQ, Zhang YM, Zhao DQ. 2003. Effects of heavy metals cadmium, lead and zinc on the survival of Carassius auratus and
Kunming Institute of Zoology (CAS), China Zoological Society Volume 34 Issues E4-5 misgurnus anguillicaudatus. Journal of Gansu Sciences, 15(1): 35-38.(in Chinese)
Wang YW, Wei YS, Liu JX. 2008. Heavy metal bioaccumulation model of aquatic organisms: An overview. Acta Scientiae Circumstantiae,28(1): 12-20. (in Chinese)
Zhang HR, Xu XX. 2009. Research on accumulate of heavy metal ions copper and lead in Cyprinus carpioi juveniles. Science and Technology of Food Industry, 30(7): 276-278. (in Chinese)
Zhang YM, Wang YJ, Yu RL, Zhou M. 2008. Effects of heavy metals on ATPase and SOD activities of hepatopancreas in Misgurnus anguillicaudatus. Journal of Gansu Sciences, 20(3): 55-59. (in Chinese)
Zhou XW, Zhu GN, Sun JH. 2002. Effects of the interaction of heavy metals on the accumulation of copper in the tissues of the fish(Carassius auratus). Journal of Zhejiang University (Agriculture &Life Sciences), 28(4): 427-430. (in Chinese)
Zhou YF, Wu W, You Y, Chen JZ. 2010. Dynamics of metallothionein in organs of Carassius auratus under combined stresses of Cd and Zn.Journal of Ecology and Rural Environment, 26(1): 63-67. (in Chinese)
- Zoological Research的其它文章
- 圍產(chǎn)期克羅米酚處理改變雄性小鼠性取向
- Description of a new Pratylenchus species from China(Tylenchida, Pratylenchidae)
- Differentiation in cranial variables among six species of Hylopetes(Sciurinae: Pteromyini)
- The first mitochondrial genome for the butterfly family Riodinidae(Abisara fylloides) and its systematic implications
- 基于類轉(zhuǎn)錄激活因子效應物(TALEs)的基因組定點操控技術(shù)
- 云南省鯉形目魚類兩新紀錄:須鯽(CARASSIOIDESACUMINATUS及矮身間吸鰍(HEMIMYZONPUMILICORPORA)

