Locally recurrent penile apocrine carcinoma initially diagnosed as metastatic adenocarcinoma of colon
Ok-Jun Lee, Seok-Joong Yun, Wun-Jae Kim, Song-Yi Choi, Ho-Chang Lee, Hyung-Geun Song, Sung-Nam Lim, Ki-Hyeong Lee, Seung-Taik Kim, Hye-Suk Han
1Department of Pathology,2Department of Urology,3Department of Internal Medicine, College of Medicine, Chungbuk National University, Cheongju 361-711, South Korea
Locally recurrent penile apocrine carcinoma initially diagnosed as metastatic adenocarcinoma of colon
Ok-Jun Lee1, Seok-Joong Yun2, Wun-Jae Kim2, Song-Yi Choi1, Ho-Chang Lee1, Hyung-Geun Song1, Sung-Nam Lim3, Ki-Hyeong Lee3, Seung-Taik Kim3, Hye-Suk Han3
1Department of Pathology,2Department of Urology,3Department of Internal Medicine, College of Medicine, Chungbuk National University, Cheongju 361-711, South Korea
Corresponding to:Hye-Suk Han, MD, PhD. Department of Internal Medicine, College of Medicine, Chungbuk National University, 776, 1Sunhwan-ro, Heungduk-gu, Cheongju 361-711, South Korea. Email: sook3529@hanamil.net.
Apocrine carcinoma is a rare malignant adnexal neoplasm. The differential diagnosis between apocrine carcinoma and cutaneous metastasis is often difficult. Here, we report a case of locally recurrent penile apocrine carcinoma initially diagnosed as metastatic adenocarcinoma of the colon. A 75-year-old man with a history of surgical resection due to sigmoid colon cancer and penile metastasis two years prior to this study presented with a nodule at the left penile base. He underwent a wide local resection of the penile mass under a suggested preoperative diagnosis of extra-mammary Paget’s disease (EMPD) associated with previous sigmoid colon cancer. However, the previously and currently resected penile masses were identified as primary apocrine carcinoma upon hematoxylin and eosin (H&E) staining and immunohistochemical staining. Although the incidence is extremely rare, both clinicians and pathologists should be alert to the possibility of synchronous double primary apocrine carcinoma in cancer patients with malignant cutaneous lesions.
Apocrine glands; carcinoma; colon cancer; penis
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/3085/3987
Introduction
Primary cutaneous apocrine carcinoma, a subtype of sweat gland carcinoma, is a rare, poorly differentiated, aggressive skin appendage tumor. The axilla and anogenital skin are the most common sites of primary disease, but lesions can also occur elsewhere on the skin (1,2). The histological features of apocrine carcinomas include adenocarcinoma with varying degrees of differentiation. Therefore, cutaneous apocrine carcinoma is indistinguishable from metastatic adenocarcinoma of the skin (mainly from the breast and gastrointestinal tracts or other visceral organs). Here, we report a rare case of synchronous double primary apocrine carcinoma, which was initially diagnosed as metastatic adenocarcinoma of the colon.
Case report
A 75-year-old man presented in March, 2012 with a 2-month history of a progressively worsening firm, nontender nodule at the left penile base and eczematous skin changes over the scrotum. He had a history of sigmoid colon cancer and penile metastasis in March, 2010. He had received low anterior resection and wide excision of a penile mass at a different hospital, followed by a 6-month course of systemic chemotherapy (comprising 5-fluorouracil, leucovorin, and oxaliplatin) until October, 2010. Physical examination revealed a raised nodular mass 2 cm × 1 cm in size at the left penile base and multiple, tiny nodular lesions at the penoscrotal junction, which were separate from the testis and epididymis. Eczematous skin changes from the penoscrotal junction to the mid-portion of the penis were also noted. A computed tomography (CT) scan of the abdomen and pelvis revealed a 1 cm × 1 cm enhancing nodule at the left penile base (Figure 1). There was no evidence of lymph node (LN) enlargement or distant metastases. Scrotal ultrasonography showed a well-definedsoft tissue lesion in the subcutaneous layer of the left penile base; both testicles and the epididymis were normal. We also conducted esophagogastroduodenoscopy, colonoscopy, a chest CT, and a positron emission tomography (PET)-CT; no further metastatic lesions or other internal malignancies were detected.
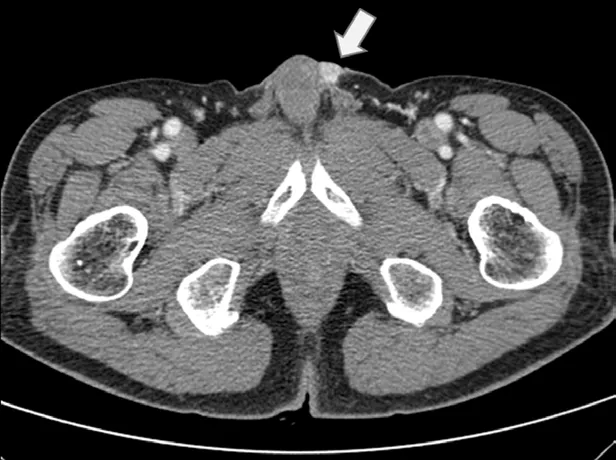
Figure 1 CT scan of pelvis showing a 1 cm × 1 cm enhancing nodule at the left penile base.
The patient underwent a wide local resection of the penile mass and surrounding eczematous skin under a suggested preoperative diagnosis of extra-mammary Paget’s disease (EMPD) associated with previous sigmoid colon cancer. Histologically, the lesion was poorly circumscribed, infiltrative, and comprised small to medium-sized abortive glands, cords, and nests extending to the surgical margins, which simulated those of invasive ductal carcinoma of the breast, but differed completely from those of colon adenocarcinoma. Microscopic examination of tumor morphology by hematoxylin and eosin (H&E) staining revealed that the tumor cells had proliferated mainly in the dermis, with pagetoid upward migration into the epidermis (Figure 2A). The tumor consisted mainly of neoplastic cells with an abundant eosinophilic cytoplasm and large nuclei containing prominent macronulceoli with mitoses (Figure 2B). There was extensive infiltration of Paget cells, which showed a pale, clear cytoplasm and large, round hyperchromatic nuclei with prominent central nucleoli, extending into the epidermis (Figure 2C). Immunohistochemical staining revealed that the tumor cells in the dermis and the intraepidermal neoplastic cells with pagetoid spread were positive for cytokeratin (CK) 7 (1:400; NeoMarkers, California, USA), epithelial membrane antigen (EMA) (1:50; Leica Microsystems, Germany), carcinoembryonic antigen (CEA) (1:100; Novocastra, Newcastle upon Tyne, UK), gross cystic disease fluid protein 15 (GCDFP-15) (1:80; Novocastra,Figure 2D), estrogen receptor (ER) (1:50; Novocastra,Figure 2E), progesterone receptor (PR) (1:200; Novocastra) and human epidermal growth factor receptor-2 (HER-2) at an intensity of 3+ (CB11, 1:200,Figure 2F), and negative for CK20 (1:100; Novocastra), human melanocyte black-45 (HMB-45) (1:100; Novocastra), S-100 (1:800; Dako, Glostrup, Demark), prostate-specific antigen (PSA) (1:200; NeoMarkers) and alpha methylacyl-CoA-racemase (AMACR) (1:200; Novocastra). A diagnosis of primary apocrine carcinoma concomitant with EMPD was made based on the morphologic and immunohistochemical findings.
Therefore, we re-examined the patient’s previously resected tumors from the sigmoid colon and penis from another hospital. The pathological and immunohistochemical findings for the previous penile mass were identical to those obtained for the current penile apocrine carcinoma. However, the microscopic findings for the colon tumor upon H&E staining differed completely from those of the penile tumor, and provided a histological diagnosis of moderately differentiated adenocarcinoma (Figure 3A). Immunohistochemical staining showed colon tumor cells positive for CK20 (1:200; Leica) (Figure 3B), but negative for CK7 (1:600; NeoMarkers) and GCDFP-15 (1:800; Dako). Accordingly, the previously resected penile tumor was diagnosed not as metastatic adenocarcinoma of the colon, but as primary apocrine carcinoma; the revised diagnosis was, therefore, synchronous double primary apocrine carcinoma and sigmoid colon cancer. The patient underwent additional radiotherapy for the genital lesions with a total radiation dose of 5,040 cGy.
Discussion
Apocrine carcinoma is a less frequent variant of skin cancer and usually presents as a single or a multi-nodular mass, or as a plaque with ulceration of the overlying skin (1,2). The differential diagnosis between apocrine carcinoma and cutaneous metastasis is often difficult because these tumors are undistinguishable both clinically and histopathologically (3-6). Patients with an underlying malignancy who show an apparently malignant skin lesion are likely to be misdiagnosed with metastasis from the underlying malignancy. Furthermore, misdiagnosis of synchronous or metachronous apocrine carcinoma as a metastasis may allowthe underlying disease to develop without proper local therapy because cutaneous metastases are classified as stage IV disease in patients with underlying primary carcinoma. Thus, a differential diagnosis of apocrine carcinoma from metastatic adenocarcinoma is very important.
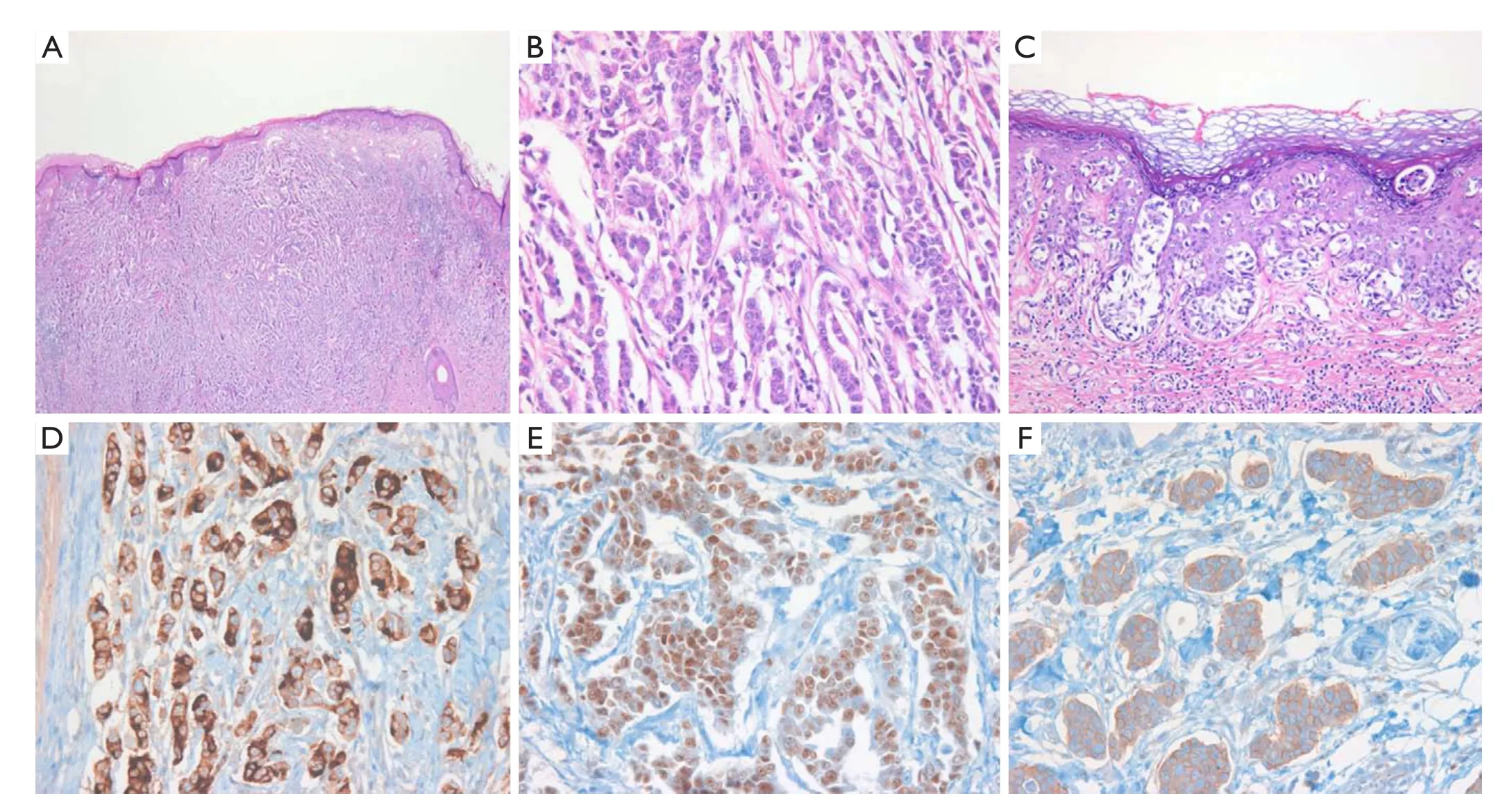
Figure 2 Histological examination and immunohistochemistry of the resected penile mass. (A) The tumor cells proliferated mainly in the dermis, forming solid pattern nests and showing pagetoid epidermal upward migration (H&E, ×100); (B) The tumor cells contain abundant eosinophilic cytoplasm, large nuclei containing prominent macronulceoli, and cytological pleomorphism (H&E, ×400); (C) There is extensive infiltration of Paget cells with pale, clear cytoplasm and large, round hyperchromatic nuclei with prominent central nucleoli, extending to the epidermis over part of the apocrine carcinoma (H&E, ×400). Immunohistochemistry shows tumor cells positive for GCDFP-15 (D), ER (E), and HER-2 (F).
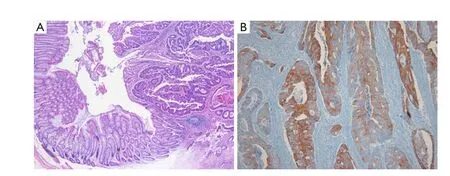
Figure 3 Histological examination and immunohistochemistry of the previously resected colon tumor. (A) Histological examination shows moderately differentiated adenocarcinoma (H&E, ×400); (B) Immunohistochemistry shows tumor cells positive for CK20 (×400).
Features that favor the diagnosis of apocrine carcinomaare the presence of neoplastic glands high in the dermis, and tumors that tend to extend into the subcutaneous tissues. The immunohistochemical details of the disease are unclear because apocrine carcinomas are rare, but there are certain immunohistochemical indications that may help to differentiate between apocrine carcinoma and cutaneous metastasis (3,4). The cells of apocrine carcinomas express low molecular weight CK, EMA, CEA, CK15, and GCDFP-15. In the past, it was thought that ER and/ or PR negativity and androgen receptor (AR) positivity were very suggestive of an apocrine phenotype. However, Robsonet al. studied a case series of cutaneous apocrine carcinomas and demonstrated that 62% were ER(+), 60% were PR(+), and 36% were AR(-) (1). In the present case, the lesion was positive for CK7, EMA, CEA, GCDFP-15, ER, PR, and HER-2, and negative for HMB-45 or S-100 (malignant melanoma markers) and PSA or AMACR (prostate carcinoma markers). Moreover, not only was tumor morphology (assessed by H&E staining) different between the penile tumor and the underlying colon cancer, but CK20 immunohistochemical staining was positive in the colon tumor and negative in the penile tumor.
Our case has several unusual presentations compared to those of previous reports of apocrine carcinoma. First, synchronous double primary apocrine carcinoma and colon cancer are extremely rare. To the best of our knowledge, only one case of apocrine carcinoma, suspected to be an axillary LN metastasis from cecal cancer, has been reported (6). Second, perineal apocrine carcinoma is usually described in women and affects the vulvar and perianal region; male penile apocrine carcinoma is extremely rare (7). Third, EMPD is characterized by the presence of varying numbers of clear cells (Paget’s cells) within the epidermis (as shown in our case); the incidence of EMPD with an underlying apocrine carcinoma is very rare (1,8-10).
This report describes a case of locally recurrent penile apocrine carcinoma initially diagnosed as metastatic adenocarcinoma of the colon. Although rare, if a cancer patient presents with a skin lesion, and if there is no metastasis from the common metastatic sites, clinicians and pathologists should be alert to the possibility of synchronous double primary apocrine carcinoma.
Acknowledgements
Written informed consent for publication of this case report and associated images was obtained from the patient. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2012-0000475).
Disclosure:The authors declare no conflict of interest.
1. Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol 2008;32:682-90.
2. Hollowell KL, Agle SC, Zervos EE, et al. Cutaneous apocrine adenocarcinoma: defining epidemiology, outcomes, and optimal therapy for a rare neoplasm. J Surg Oncol 2012;105:415-9.
3. Fernandez-Flores A. Primary cutaneous apocrine carcinoma versus metastasis, a plea to the dermatopathology community. Am J Dermatopathol 2010;32:853-4.
4. Wako M, Nishimaki K, Kawamura N, et al. Mucinous carcinoma of the skin with apocrine-type differentiation: immunohistochemical studies. Am J Dermatopathol 2003;25:66-70.
5. Terada T, Sato Y, Furukawa K, et al. Primary cutaneous mucinous carcinoma initially diagnosed as metastatic adenocarcinoma. Tohoku J Exp Med 2004;203:345-8.
6. Ogino T, Noura S, Ohue M, et al. A case of apocrine adenocarcinoma suspected as an axillary lymph node metastasis from cecal cancer. Gan To Kagaku Ryoho 2006;33:1968-70.
7. Treiyer A, Haben B, R?ttger P, et al. First male apocrine genital carcinoma mimicking a penile cancer. Urology 2008;71:e11-2.
8. Seo SH, Shin DH, Sung HW. Apocrine Carcinoma of the Groin Possibly Associated with Extramammary Paget’s Disease. Ann Dermatol 2011;23:519-22.
9. Usui K, Ochiai T, Abe I, et al. Apocrine gland carcinoma of the mammary skin concomitant with pagetoid phenomenon. J Dermatol 2010;37:350-4.
10. Miyamoto T, Adachi K, Fujishima M. Axillary apocrine carcinoma with Paget’s disease and apocrine naevus. Clin Exp Dermatol 2009;34:e110-3.
Cite this article as:Lee OJ, Yun SJ, Kim WJ, Choi SY, Lee HC, Song HG, Lim SN, Lee KH, Kim ST, Han HS. Locally recurrent penile apocrine carcinoma initially diagnosed as metastatic adenocarcinoma of colon. Chin J Cancer Res 2013;25(6):777-780. doi: 10.3978/j.issn.1000-9604.2013.12.11

10.3978/j.issn.1000-9604.2013.12.11
Submitted Feb 24, 2013. Accepted for publication Jul 22, 2013.
 Chinese Journal of Cancer Research2013年6期
Chinese Journal of Cancer Research2013年6期
- Chinese Journal of Cancer Research的其它文章
- Clinicopathological and prognostic role of MMP-9 in esophageal squamous cell carcinoma: a meta-analysis
- A “Stem Cells in Cancer” special issue in Translational Cancer Research
- Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer
- The effect of beta-elemene on alpha-tubulin polymerization in human hepatoma HepG2 cells
- Spectral CT imaging as a new quantitative tool? Assessment of perfusion defects of pulmonary parenchyma in patients with lung cancer
- Clinical antiangiogenic effect of recombinant adenovirus-p53 combined with hyperthermia for advanced cancer
