Solubilities of Nizatidine in Methanol + Water, Ethanol + Water and i-Propanol + Water from 273.15 to 303.15 K*
LI Yin (李音) and Lü Xiuyang (呂秀陽)**
Key Laboratory of Biomass Chemical Engineering of Ministry of Education, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
1 INTRODUCTION
Nizatidine (N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine) (CAS 76963-41-2) is a specific H2-receptor antagonist and has been proved to be effective in the treatment of duodenal ulceration [1]. Crystallization in ethanol or acetone is employed in the final step of nizatidine preparation [2, 3]. Moreover, environmentally friendly processes using water, water + ethanol mixtures as solvents to crystallize nizatidine are under investigation.
The cosolvency, mixing a permissible nontoxic organic solvent with water, is one of the most common techniques to increase the aqueous solubility of drugs,and the mixtures could be used in purification and formulation processes of drugs. Due to its importance,the cosolvency in the water-cosolvent mixtures attracts more attentions nowadays. The cosolvency models and their advantages and limitations were reviewed recently [4, 5].
In our previous work, the solubilities of nizatidine in water, methanol, ethanol, acetone and ethyl acetate were determined in the temperature range from 273.15 K to 343.15 K [6], and it was found that the presence of a small amount ethanol significantly changes the solubility of nizatidine in ethanol-water mixtures. Therefore, solubility measurement of nizatidine in mixtures is essential for developing crystallization processes. It can also provide basic data for the development of cosolvency theory and models.
In this study, the solubilities of nizatidine in methanol + water, ethanol + water andi-propanol +water mixtures are determined in the temperature range from 273.15 to 303.15 K. Upon comparing the correlations of different cosolvency models, the general single model (GSM) [4, 7] is adopted for correlating the experimental data.
2 EXPERIMENTAL
2.1 Chemicals
Nizatidine (>99.5% purity) was obtained from Hengdian Group Jiayuan Chemistry Industry Co., Ltd,China. Analytical reagent (AR) grade methanol (>99.5%purity), ethanol (>99.7% purity) andi-propanol (>99.7%purity) were obtained from Sinopharm Chemical Reagent Co., Ltd. High-performance liquid chromatograph(HPLC) grade methanol and acetonitrile were obtained from Merck. All chemicals were used as received.
2.2 Apparatus and procedure
The solubilities of nizatidine in methanol + water,ethanol + water andi-propanol + water mixtures were measured by a static analytical method [8, 9] similar to that described in our previous work [6, 10-12]. Binary solvents with certain mass fraction of methanol, ethanol, andi-propanol were prepared. Excess solute and certain binary solvent were put into a jacketed glass vessel placed in a precision water bath and maintained the desired temperature within the uncertainty of±0.05 K. 9 h of stirring and 9 h of settling were employed to ensure the equilibrium. Samples were taken with a Pasteur pipette and analyzed by HPLC. A condenser with a balloon on top was connected to the vessel to prevent the solvent from evaporating and keep the constant composition of the binary solvent.
2.3 Analysis
Six samples were collected and analyzed at each temperature. The analysis process was similar to our previous work [6]. About 0.5 g of saturated solution was collected, put into a volumetric flask with stopper,weighed, diluted with water to a certain volume, and then the composition of the pretreated sample was directly determined by reverse-phase HPLC (Agilent 1100 series) with external standard method. The HPLC column was an Phenomenex-C18(250×4.6 mm, 5 μ).0.02 mol·L-1disodium hydrogen phosphate aqueous solution, acetonitrile, methanol, and triethylamine in a volume ratio of 77.95∶10∶10∶0.05 were used as the mobile phase at a flow rate of 0.8 ml·min-1[13].The column temperature was 303.15 K and the UV wavelength was set at 254 nm. The linear range was(0.03 to 0.88) mg·ml-1with correlation coefficient close to 1 and RSD (relative standard deviation) (n=6)at 0.0008.
3 RESULTS AND DISCUSSION
The average experimental mass fraction solubilities of nizatidine in methanol + water, ethanol + water andi-propanol + water mixtures are listed in Table 1,together with the standard uncertainty (k=1). The solubilities of nizatidine in methanol, ethanol and water from our previous work [6] are also listed in Table 1 for comparison. The solubility data are depicted as a function of organic solvent concentration in Figs. 1-3.
The GSM is proposed to correlate the solubility of nizatidine.

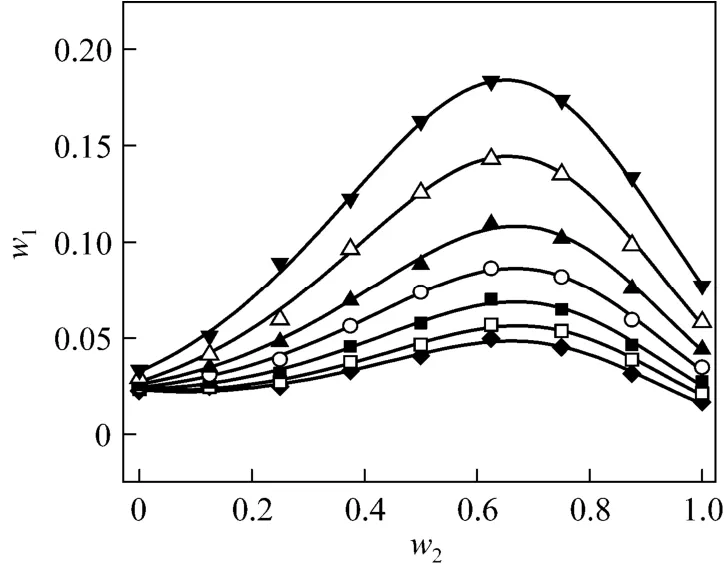
Figure 1 Solubilities of nizatidine in methanol + water mixtures▼ 303.15 K; △ 298.15 K; ▲ 293.15 K; ○ 288.15 K; ■ 283.15 K;□ 278.15 K; ◆ 273.15 K; calculated from Eq. (1)

Figure 2 Solubilities of nizatidine in ethanol + water mixtures▼ 303.15 K; △ 298.15 K; ▲ 293.15 K; ○ 288.15 K; ■ 283.15 K;□ 278.15 K; ◆ 273.15 K; calculated from Eq. (1)

Figure 3 Solubilities of nizatidine in i-propanol + water mixtures▼ 303.15 K; △ 298.15 K; ▲ 293.15 K; ○ 288.15 K; ■ 283.15 K;□ 278.15 K; ◆ 273.15 K; calculated from Eq. (1)
wherew1is mass fraction solubility of nizatidine,w2is mass fraction of organic solvent in the absence of nizatidine,K0,K1,K2andK3are parameters.
The values ofK0,K1,K2andK3in Eq. (1) for methanol + water, ethanol + water andi-propanol +water mixtures are presented in Table 2, together with the root-mean-square deviation defined by
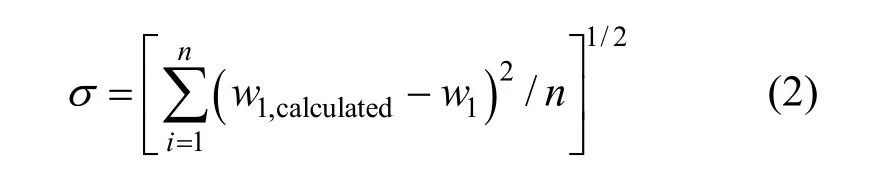
wherew1is experimental mass fraction of nizatidine,w1,calculatedis the calculated mass fraction of nizatidine,andnis the number of data in each set. The calculated curves for methanol + water, ethanol + water andi-propanol + water mixtures from Eq. (1) are also shown in Figs. 1-3, suggesting that the equation fits the data very well.

Table 2 Parameters in Eq. (1) for methanol + water, ethanol + water and i-propanol + water mixtures, together with σ

Table 3 Parameters in Eq. (3) for methanol + water, ethanol + water and i-propanol + water mixtures, together with R2

Table 4 Mass fraction of organic solvents and mass fraction solubilities of nizatidine at the maximum points calculated with Eq. (1) for methanol + water, ethanol + water and i-propanol + water mixtures
Furthermore, a simple polynomial equation can be used to correlate the parameters of Eq. (1) as functions of temperature,

whereTis the absolute temperature,a,bandcare parameters,c=0 wheni=1, 2, 3.
The valuesa,bandcin Eq. (3) are presented in Table 3, and the correlation coefficients are greater than 0.98.
From the data, we can see that the concentration of organic solvent has a significant effect on the solubility of nizatidine, and the curves are similar for the three systems. At the same temperature, the solubilities of nizatidine increase with the mass fraction of organic solvent, reach the maximum values, then decrease.However, the curves reach the peaks at different concentrations of organic solvents for the three systems.
Mass fraction of organic solvents and mass fraction solubilities of nizatidine at the maximum points calculated with Eq. (1) for the three systems are listed in Table 4. The dielectric constants of water, methanol,ethanol andi-propanol are 79.7, 32.6, 22.4, and 18.3 at 293.15 K, respectively [14]. The mass fraction of organic solvents at the maximum point increases as polarity of the organic solvent increases.
4 CONCLUSIONS
The solubilities of nizatidine in methanol + water,ethanol + water andi-propanol + water mixtures were determined in the temperature range from 273.15 to 303.15 K by a static analytical method, which are essential for the development of crystallization processes and formulation processes. The GSM was used to correlate the experimental data, which fits the data well.
NOMENCLATURE

1 Stephenson, G.A., Wozniak, T.J., Stowell, J.G., Byrn, S.R., “Nizatidine,N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1, 1-ethenediamine.”,J.Mol.Struct., 380, 93-100(1996).
2 Manning, H.W., “Process for preparing nizatidine”, US Pat.,5700945 (1997).
3 Cornwall, P., “Process for the preparation of nizatidine”, US Pat.,6069256 (2000).
4 Jouyban, A., Handbook of Solubility Data for Pharmaceuticals, CRC Press, Boca Raton (2009).
5 Jouyban, A., “Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures”,J.Pharm.Pharmaceut.Sci., 11, 32-58 (2008).
6 Li, Y., Lü, X.Y., “Solubilities of nizatidine in water, methanol, ethanol, acetone and ethyl acetate from (273.15 to 343.15) K”,J.Chem.Eng.Data, 55, 4019-4020(2010).
7 Barzegar-Jalali, M., Jouyban-Gharamaleki, A., “A general model from theoretical cosolvency models”,Int.J.Pharm., 152, 247-250(1997).
8 Lu, Y.C., Lin, Q., Luo, G.S., Dai, Y.Y., “Solubility of emodin in alchhols”,Chin.J.Chem.Eng.,17, 251-253 (2009).
9 Shao, J., Zeng, Z.X., Xue, W.L., Wu, C., “Experimental measurement and correlation of the solubility of methy-acetyl-naphthalene inn-heptane,n-octane, andn-dodecane”,Chin.J.Chem.Eng.,14,780-783 (2006).
10 Lu, L.L., Lü, X.Y., “Solubilities of gallic acid and its esters in water”,J.Chem.Eng.Data, 52, 37-39 (2007).
11 Lu, L.L., Lü, X.Y., “Solubilities of polyhydroxybenzophenones in an ethanol + water mixture from (293.15 to 343.15) K”,J.Chem.Eng.Data, 53, 1996-1998 (2008).
12 Zheng, X.F., Lü, X.Y., “Measurement and correlation of solubilities of asiaticoside in water, methanol, ethanol,n-propanol,n-butanol,and a methanol + water mixture from (278.15 to 343.15) K”,J.Chem.Eng.Data, 56, 674-677 (2011).
13 Yusuf, A., Dgither, S.A., Hammami, M.M., “Validation of a new high-performance liquid chromatography assay for nizatidine”,Ther.DrugMonit., 28, 232-236 (2006).
14 Smallwood, I.M., Handbook of Organic Solvent Properties, Elsevier,London (1996).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
