Measurements of Conductivity for Low Concentration Strongelectrolytes in Organic Solvents (I) LiBr, LiCl, and LiNO3 in Alcohols
CHEN Hong (陳紅), WANG Lisheng (王利生)*, JIANG Bo (姜波) and LI Miyi (李彌異)
School of Chemical Engineering and the Environment, Beijing Institute of Technology, Beijing 100081, China
1 INTRODUCTION
Thermodynamic properties of electrolyte solutions containing organic solvents are important in chemical engineering calculations. The design of extractive distillation [1], food processes [2, 3], and brine preparation processes [4] requires thermodynamic property data of electrolyte solutions. The determination of mean ionic activity coefficients of electrolytes is essential in developing salt industry, sea-water desalination process and waste water treatment. Moreover, absorption refrigeration machines and heat pumps are receiving more attention in the refrigeration and air-condition industry. The efficiency of an absorption refrigeration machine and heat pump cycles is largely dependent on the physical and chemical properties of heat transfer fluids. The solutions of LiBr + methanol and LiCl +methanol can be used to replace aqueous solutions at temperatures below the freezing-point of water [5].The use of solutions with different lithium salt in alcohol as heat transfer fluids in power plants facilities the development of new, more efficient absorption cycles.
This study is an effort to extend the information on the physical properties of binary solutions of lithium salts in alcohols. The measurement of conductivity is a technique that yields not only values of mobility of ions in an electrolyte, but also thermodynamic parameters such as activity and osmotic coefficients at low concentrations. The experiments are carried out in the temperature rangeT=298.15-323.15 K at concentrations fromc=0.01 to 0.08 mol·L-1. From these data the mean activity coefficients of LiBr, LiCl, and LiNO3in methanol, ethanol, 1-propanol, and 2-propanol are evaluated.
2 EXPERIMENTAL
2.1 Materials
LiBr and LiCl were purchased from Beijing Chemical Reagents Company. LiNO3was purchased from Tianjin Fuchen Chemical Reagent Company. All the salts were analytical reagents. All the solvents were chromatographically grade reagents with mass fraction purities higher than 99.9%. The organic solvents were used without further purification.
2.2 Apparatus
Conductivity was measured by using conductivity meter (Mettler-Toledo Instrument Co., Ltd. type FE30) with ZNWH-II intelligent temperature control instrument (Henan Aibote Science and Technology Development Co., Ltd) and an SYP glass thermo-stated bath (Nanjing Sangli Electronic Equipment Factory).The apparatus used in this work is shown in Fig. 1.
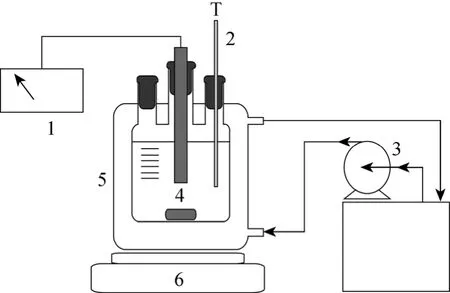
Figure 1 The apparatus for conductivity measurement1—conductivity meter; 2—thermometer; 3—temperaturecontrolled bath and pump; 4—magnetic stirring rod; 5—jacketed equilibrium cell; 6—magnetic stirrer
2.3 Procedure of measurement
The calibration was needed before using the FE30 conductivity meter. The standard solution provided by manufacture was prepared at 298.15 K. The electrode was put into the standard solution, and the systematic deviation was removed when the instrument became stable and the value of the instrument using the standard conductivity solution showed consistency with the default setting.
For measuring the conductivity of salt-organic systems, the salt was re-crystallized and dried in an oven at 420 K for 48 h. In a nitrogen protected glove box, the desired salt and solvent were weighed using an analytical balance (type TG328B, Shanghai Balance Instrument Works Co., with an uncertainty of±0.01 mg) and put into a scaled cell with thermo-stated jacket. The estimated relative uncertainty on concentration of electrolyte based on error analysis and repeated observations was within 2%. After 2 h stirring to achieve stable condition, the FE30-electrical conductivity meter was inserted into the solution and the value was recorded. The uncertainty of the conductivity is ±0.02 μS·cm-1.
3 RESULTS AND DISCUSSION
3.1 Conductivity and correlation
The measured data of conductivityκin S?cm-1of the electrolyte solutions in a low concentration range fromc=0.01 to 0.08 mol·L-1at different temperatures are listed in Tables 1-3. The conductivities of the puresolvents were also measured, listed in Table 4. The molar conductivityΛin S·cm2·mol-1can be calculated as follows

Table 1 Conductivity κ of LiBr in pure solvent at different concentrations and temperatures
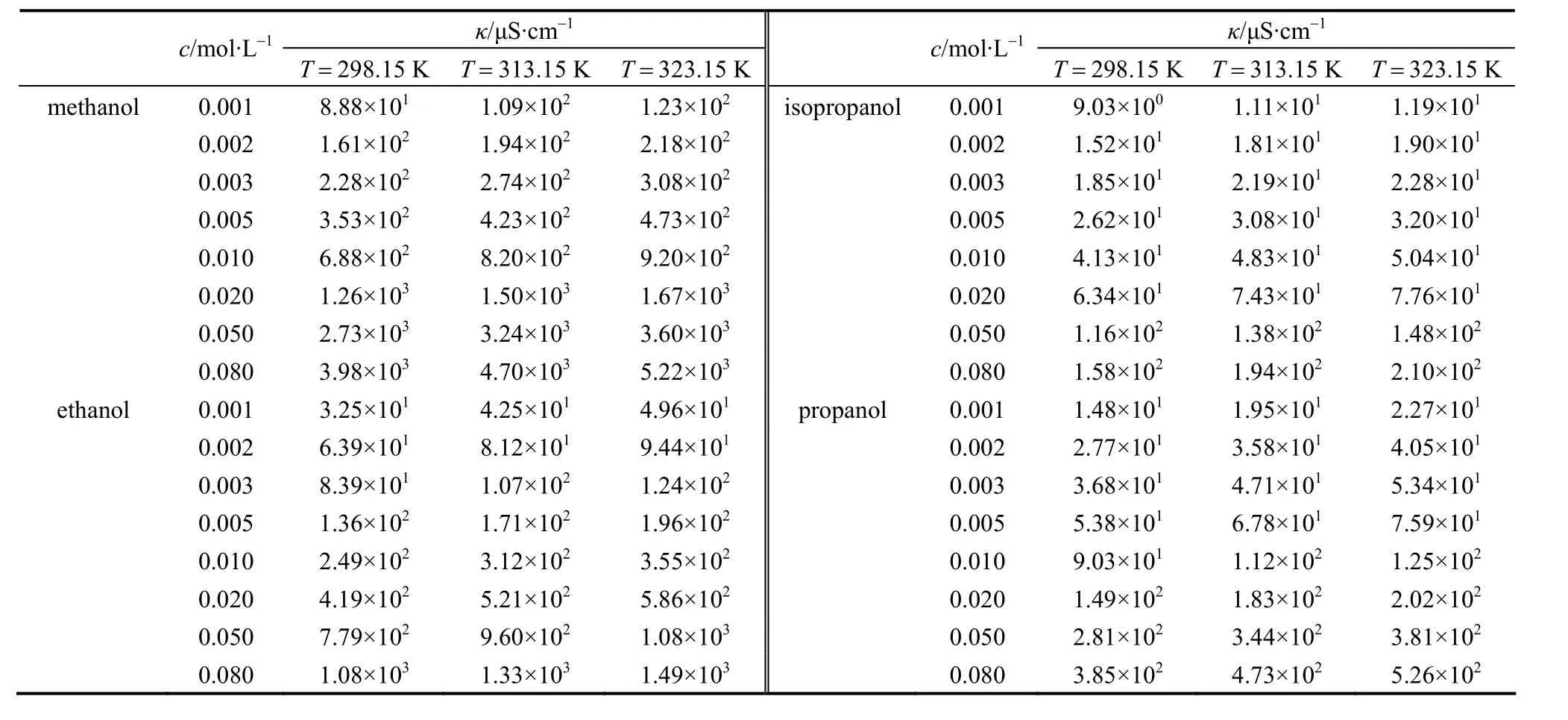
Table 2 Conductivity κ of LiCl in pure solvent at different concentrations and temperatures

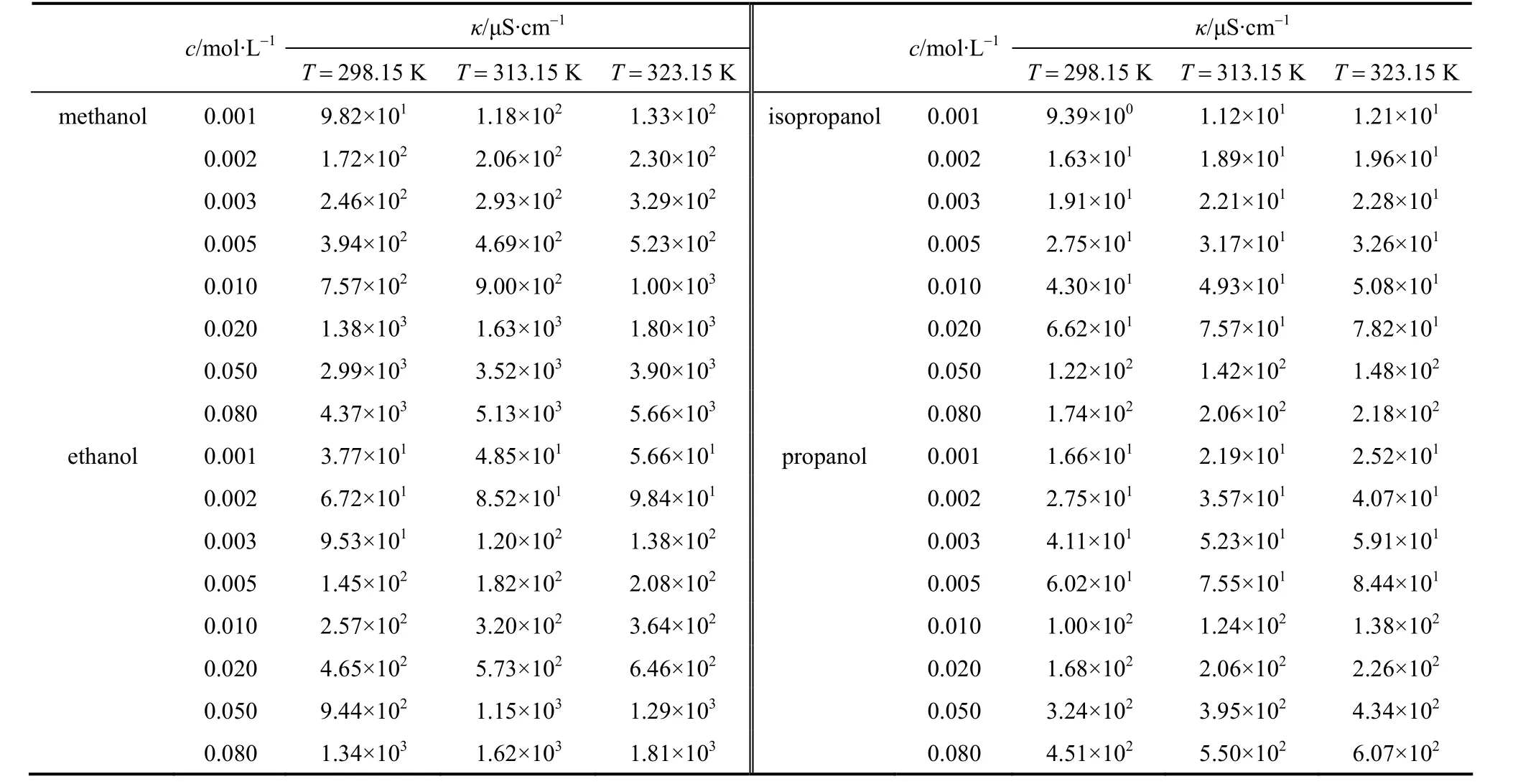
Table 3 Conductivity κ of LiNO3 in pure solvent at different concentrations and temperatures
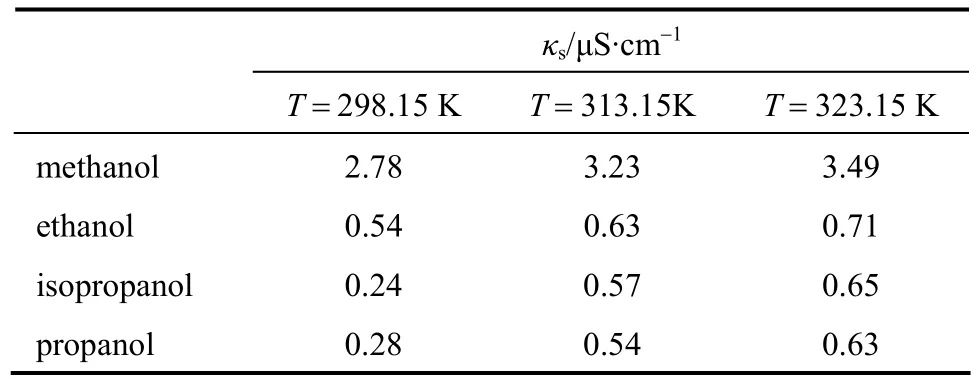
Table 4 Conductivity κs of pure solvent at different temperatures
The concentration dependence of molar conductivity is analyzed with Foss-Chen-Justice (FCJ) equation [6]
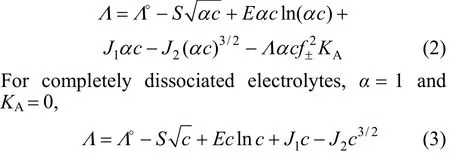
The fitting parameters are the limiting molar conductivityΛ°andS,E,J1,J2. Figs. 2-4 show that the measured molar conductivitiesΛ°vs. molar concentrationcare fitted well with Eq. (3). The conductivity of different salts in all of the investigated alcohols decreases rapidly with the increase of concentration in the low concentration range. The obtained values of limiting molar conductivityΛ°and other parameters are listed in Tables 5-7.
3.2 Activity coefficients from conductivity data
To describe the mean ion activity coefficients,many models had been developed in the past, and many researchers had made great efforts on this topic[7-11]. The mean activity coefficient of solutef±, on a molar scale is defined as
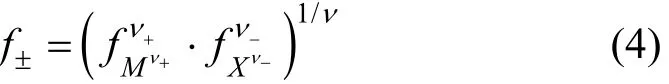
whereν+andν-are the stoichiometric coefficients,andν=ν++ν-. At low concentrations (c≤0.1 mol·L-1),f±can be represented by the well-known Debye-Hückel equation:
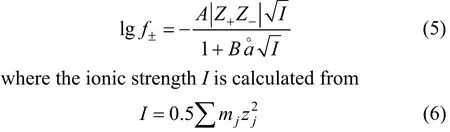
wheremjis the molality of ion, andzjis the charge number of ion. The molar conductivity of solution can be represented by Onsager equation [12, 13]
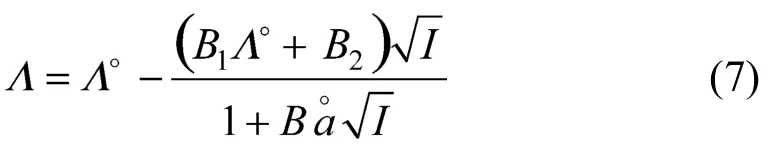

Figure 2 Electrical conductivity of LiBr in alcohols measured in this work▲ 298.15 K; ◆ 313.15 K; ■ 323.15 K; FCJ fitting

Figure 3 Electrical conductivity of LiCl in alcohols measured in this work▲ 298.15 K; ◆ 313.15 K; ■ 323.15 K; FCJ fitting
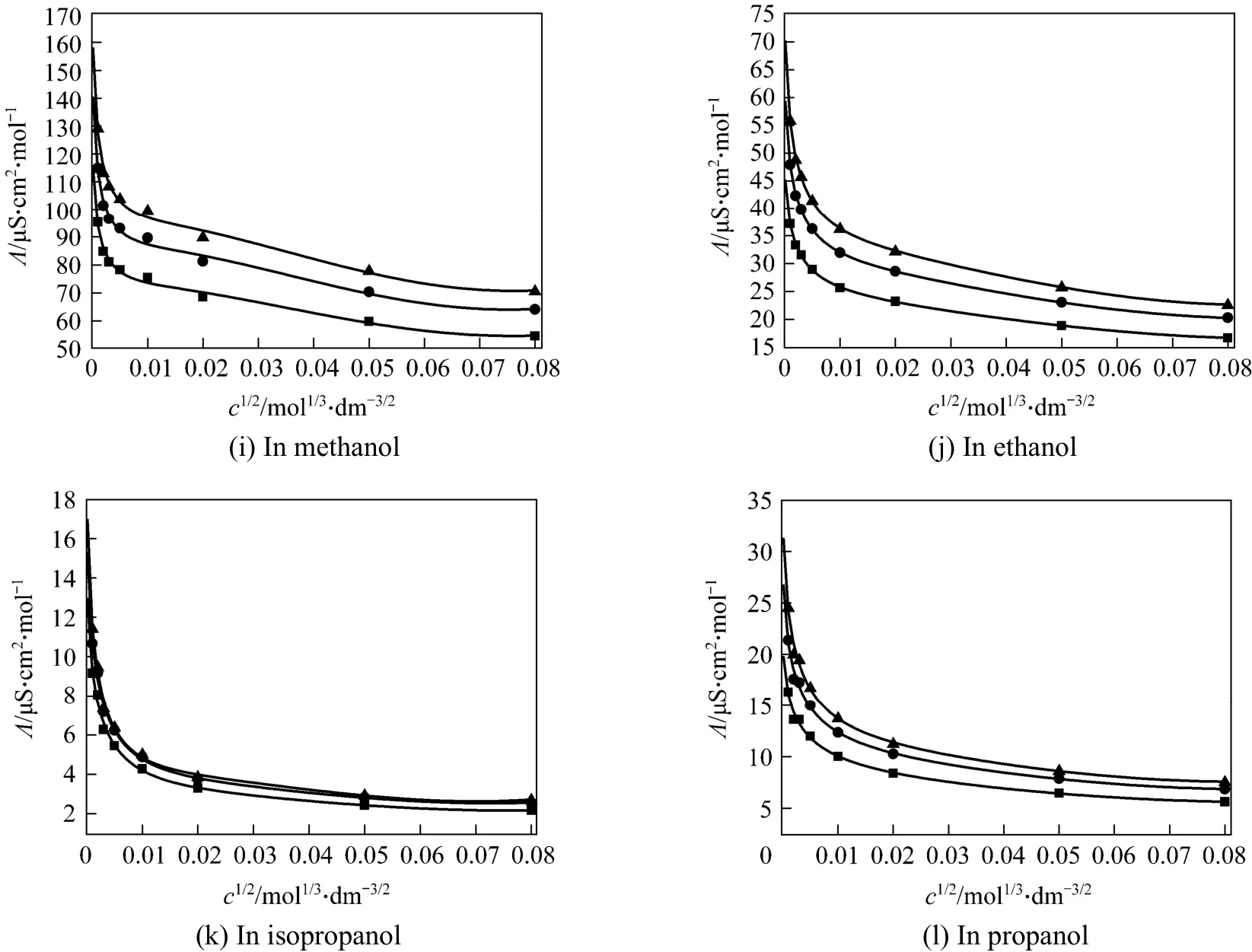
Figure 4 Electrical conductivity of LiNO3 in alcohols measured in this work▲ 298.15 K; ◆ 313.15 K; ■ 323.15 K; FCJ fitting

Table 5 Limiting molar conductivities and S, E, J1, J2 of LiBr in pure solvents
Then the activity coefficients can be rearranged with Eqs. (5) and (7) as
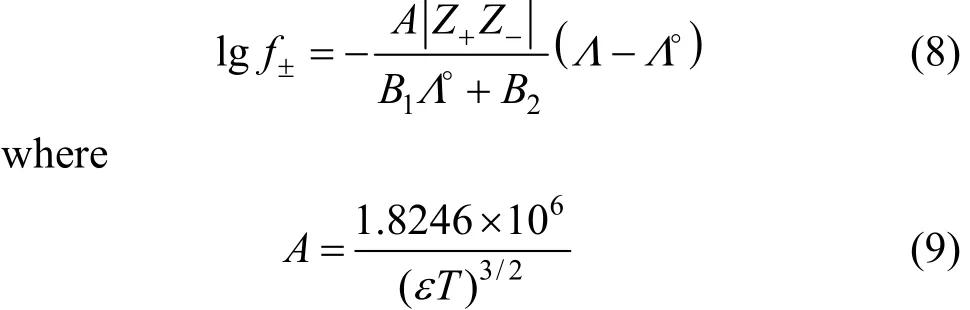
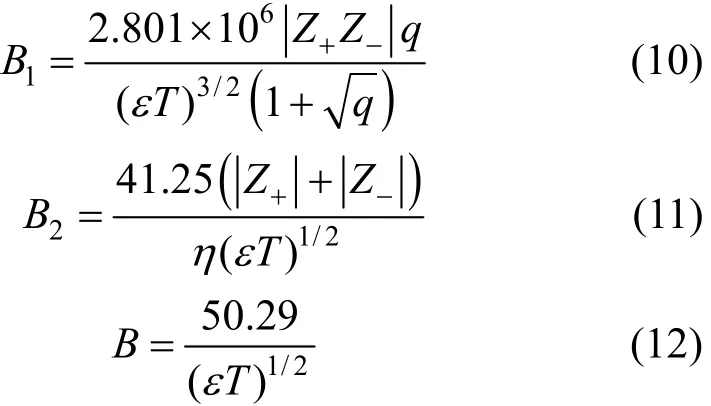

Table 6 Limiting molar conductivities and S, E, J1, J2 of LiCl in pure solvents
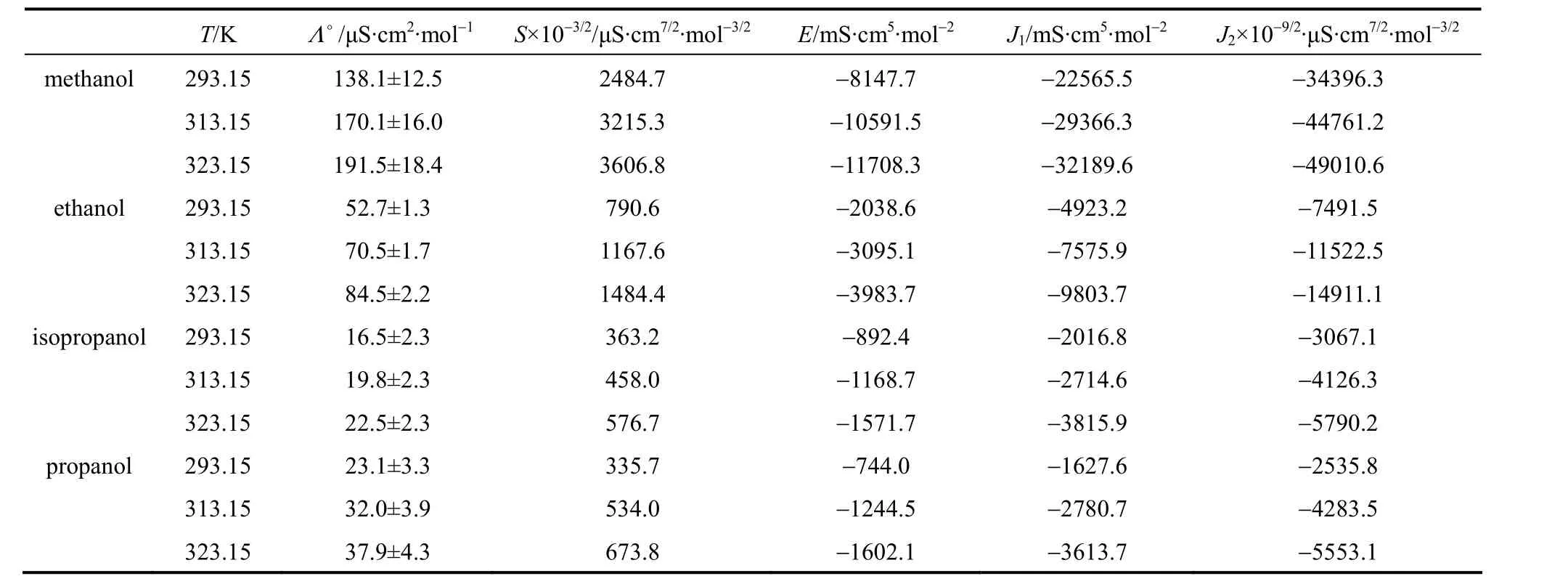
Table 7 Limiting molar conductivities and S, E, J1, J2 of LiNO3 in pure solvents
For a 1-1 electrolyte,
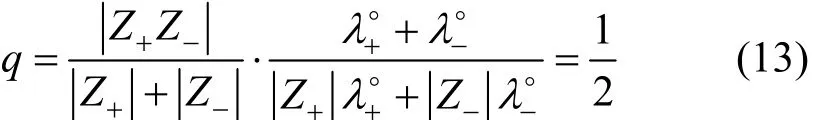
In above equations,f±is the rational activity coefficient of electrolyte,Z+andZ-are algebraic charge number of cation and anion, respectively,Iis the ionic strength in mol·kg-1,ΛandΛ°are molar conductivity and limiting molar conductivity of electrolyte, respectively,λ+°andλ-°are limiting molar conductivity of cation and anion respectively,is a parameter related to ion diameter,ηandεare viscosity and dielectric constants of solvent, respectively. The values ofηandεare obtained from literature [14-16].
With a parameterddefined as

Eq. (8) can be simplified as

A usual concentration scale of electrolyte solution is the molality scale (mol·kg-1). The standard state of ionjin the solution is defined as the hypothetical infinite dilute solution in solventsat unit concentration. Based on molality scale, the complete expression of the mean activity coefficientγ± of the electrolyte can be obtained from

wheremandMsare the molality of solution and molar mass of solvent, respectively.
The equation of mean ion activity coefficient is rearranged with Eqs. (15) and (16) as
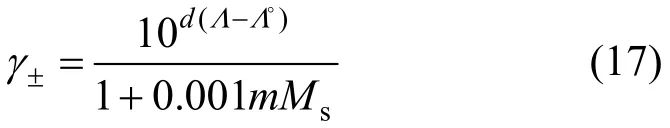
where molalitymis calculated from the experimental molar concentrationcby

In this work, the liquid density and viscosity of the solvents are taken from literature [14]. With Eq. (17),mean ionic activity coefficients of salts in organic solvents can be calculated from the measured conductivity data and limiting conductivity. The results are listed in Tables 8-10. It should be noted that Eq. (17)is only applicable to non-associated electrolyte solutions and electrolyte solutions with concentration less than 0.1 mol·L-1. The activity coefficients obtained in this work are compared with reference data [5, 17-19](determined by different method), as shown in Figs. 5 and 6 for different systems. The trend of the activity coefficients obtained in this work is in good agreement with those literature data.

Table 8 Activity coefficient of LiBr in pure solvent at different concentrations and temperatures
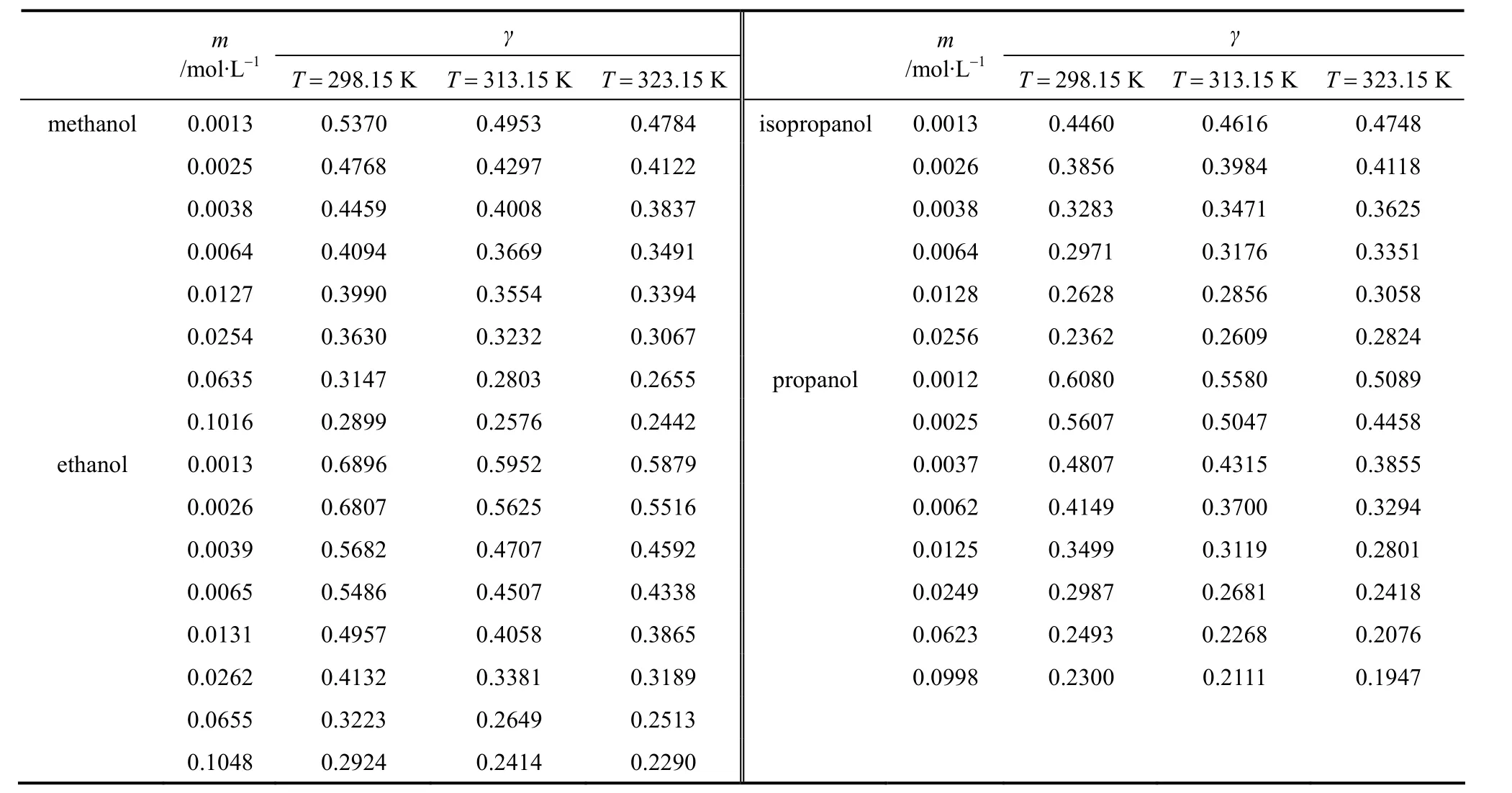
Table 9 Activity coefficient of LiCl in pure solvent at different concentrations and temperatures
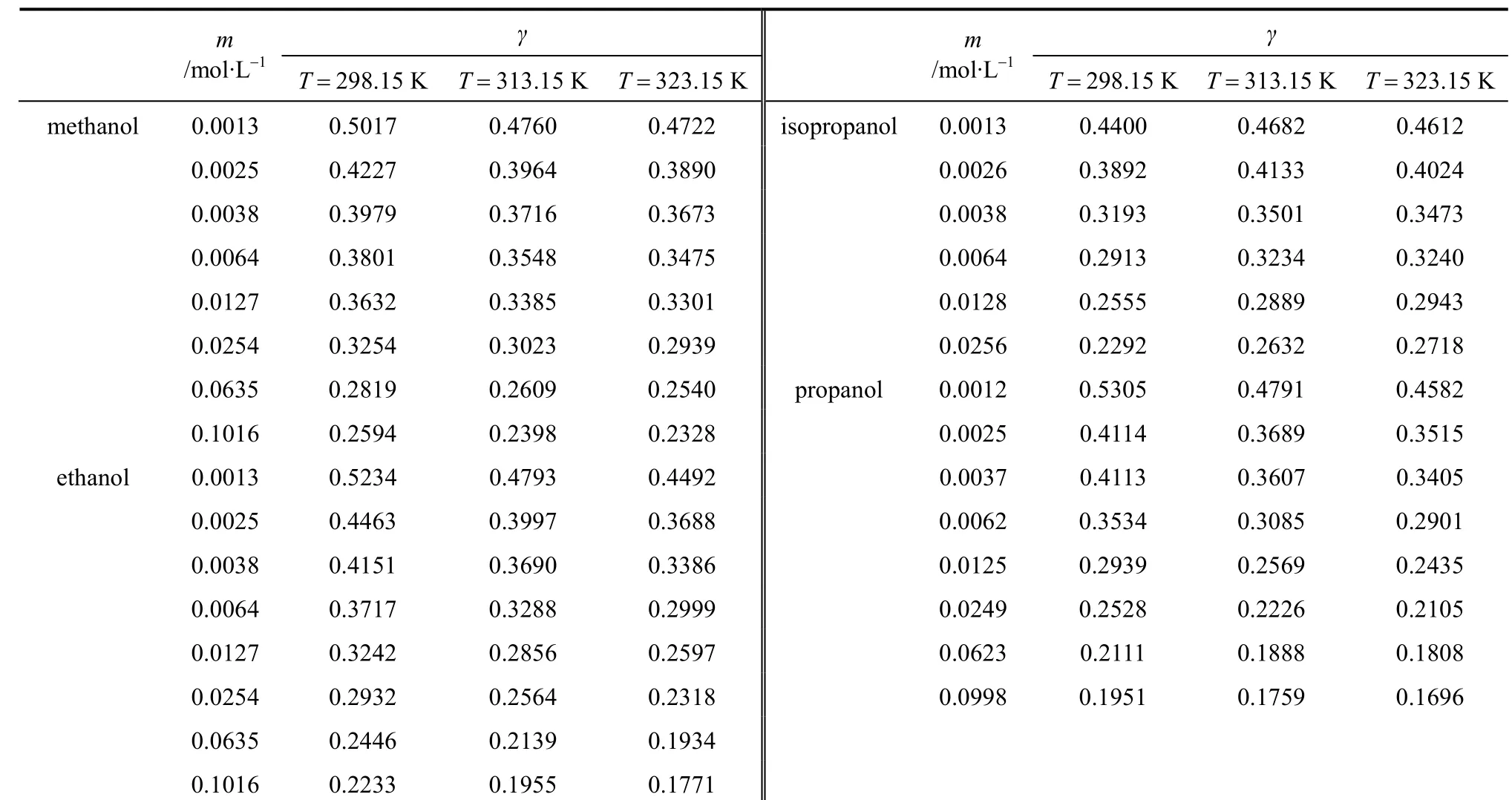
Table 10 Activity coefficient of LiNO3 in pure solvent at different concentrations and temperatures
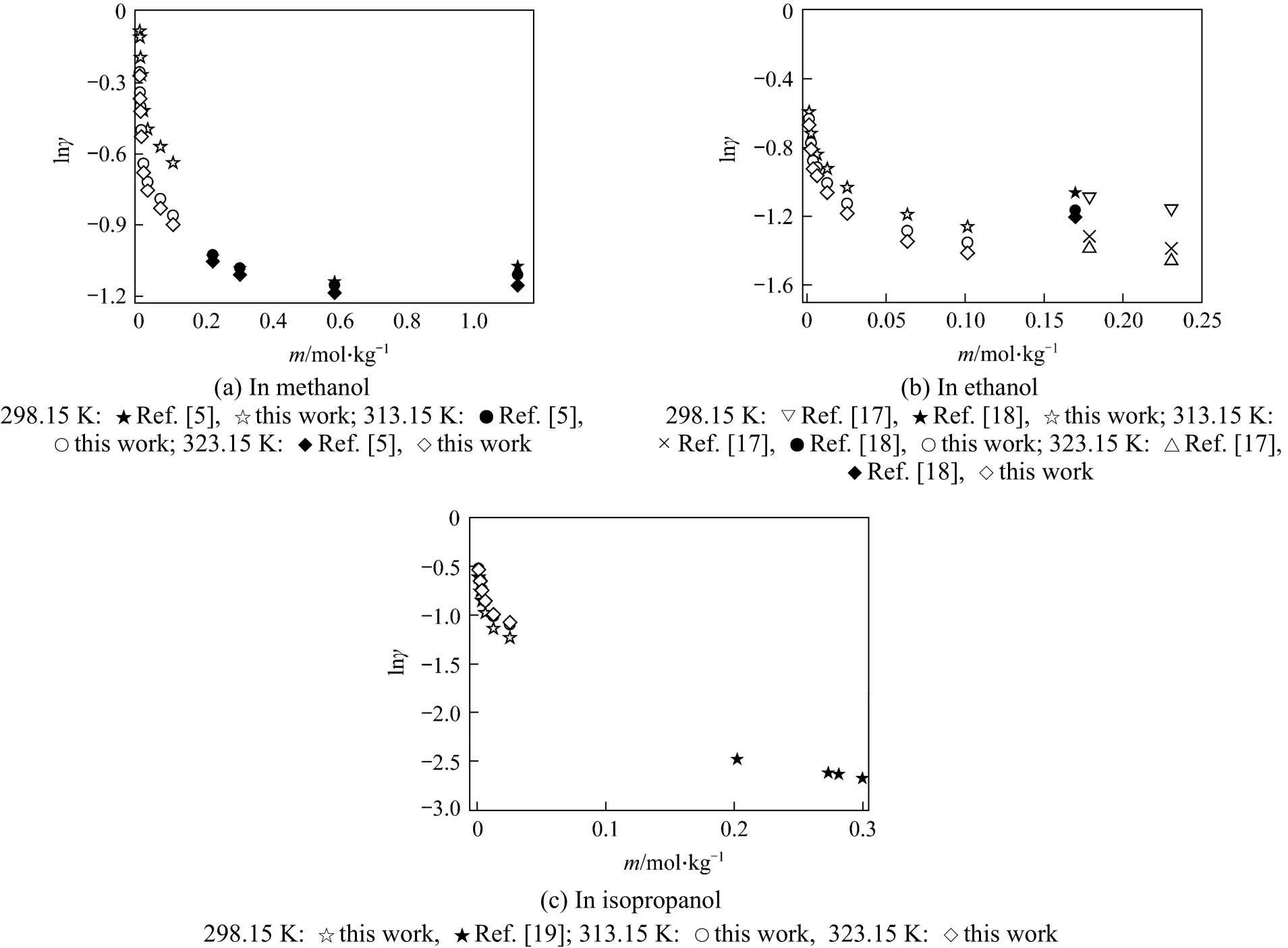
Figure 5 Comparison of activity coefficient of LiBr in alcohols at different concentrations and temperatures
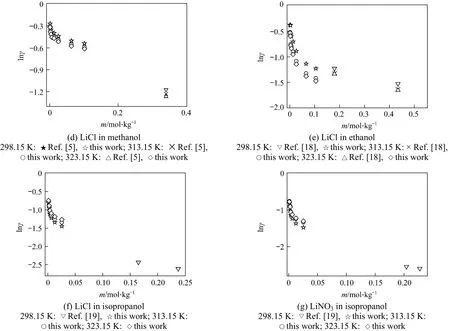
Figure 6 Comparison of activity coefficient of LiCl and LiNO3 in alcohols at different concentrations and temperatures
4 CONCLUSIONS
The conductivity of strong electrolytes in organic solvents is measured with a conductivity meter. The mean ionic activity coefficients of electrolytes are obtained from the conductivity data of the salts in the organic solvents in a low concentration range. This method is simple and convenient with satisfactory accuracy.
NOMENCLATURE
adistance of closest approach of bare ions
parameter related to ion diameter
cmolar concentration of solution, mol·L-1
f±rational activity coefficient of solution
KAassociation constant
Mmolecule mass of salt, kg·mol-1
MSmolecule mass of solvent, kg·mol-1
mmolality of solution, mol·kg-1
S,E,J1,J2parameters in FCJ equation
Tabsolute temperature, K
Z+algebraic charge number of cation
Z-algebraic charge number of anion
αdissociation constant
γ±m(xù)ean ionic activity coefficient
εdielectric constant of solution, F·m-1
ηviscosity of solution, Pa·s
κconductivity of solution, μS·cm-1
κsconductivity of pure solvent, μS·cm-1
Λmolar conductivity of solution, μS·cm2·mol-1
Λ°limiting molar conductivity of solution, μS·cm2·mol-1
0λ+limiting molar conductivity of cation, μS·cm2·mol-1
0λ-limiting molar conductivity of anion, μS·cm2·mol-1
1 Fu, J.Q., “Simulation of salt-containing extractive distillation for the system of ethanol/water/ethanediol/KAc (2) Simulation of salt-containing extractive distillation”,Ind.Eng.Chem.Res., 43, 1279-1284 (2004).
2 Sucman, E., Bednar, J., “Determination of chlorides in foods using ion-selective electrodes-a review”,Can.J.Anal.Sci.Spectrosc., 47,66-71 (2002).
3 Perez-Olmos, R., Yoldi, I., Ruiz, M.P., Merino, J.M., “Potentiometric determination of nitrite in meat products using a nitrite-selective electrode”,Anal.Sci., 14, 1001-1007 (1998).
4 J?ssang, A., Stange, E., “A new predictive activity model for aqueous salt solutions”,Fluid PhaseEquilib., 181, 33-41 (2001).
5 Safarov, J.T., “Study of thermodynamic properties of binary solutions of lithium bromide or lithium chloride with methanol”,Fluid Phase Equilib., 236, 87-95 (2005).
6 Fernader-Prini, R., “Chapter 5”, In: The Physical Chemistry of Or-ganic Solvent Systems, Covington, A.K., Dickinson, T., eds., Plenum Press, New York (1973).
7 Robinson, R.A., Stokes, R.H., Electrolyte Solutions, 2nd edition;Butterworths, London (1959).
8 Pitzer, K. S. “Thermodynamics of electrolytes (I) Theoretical and general equations”, J. Phys. Chem., 77, 268-273 (1973).
9 Xu, Y., Liu, G., Hu, Y., “Molecular thermodynamics of gas solubility(II) Henry’s constants of gases in polar solvents and 1-1 type electrolyte solutions”, Chin. J. Chem. Eng., 3, 163-184 (1988).
10 Zuo, Y., Guo, T., “An equation of state for aqueous electrolyte systems-prediction of the solubility of natural gas in formation water”,Chin. J. Chem. Eng., 2, 126-141 (1991).
11 Liu, Z., Liu, Y., Hu, Y., Zeng, P., Fan, S., Liang, D., “Prediction of activity coefficients for mixed aqueous electrolyte solutions from the data of their binary solutions”, Chin. J. Chem. Eng., 14, 494-504(2006).
12 Onsager, L., “The theory of electrolytes (I)”, Phys. Z., 27, 388-392(1926).
13 Onsager, L., “The theory of electrolytes (II)”, Phys. Z., 28, 277-298(1927).
14 Yaws, C.L., Chemical Properties Handbook, McGraw-Hill, USA(1999).
15 Shirke, R.M., Chaudhari, A., More, N.M., Patil, P.B., “Dielectric measurements on methyl acetate + alcohol mixtures at (288, 298, 308,and 318) K using the time domain technique”, J. Chem. Eng. Data,45, 917-919 (2000).
16 Zhuravlev, V.I., Durov, V., A., Usacheva, T.M., Shakhparonov, M.I.,“Dielectric properties of 1,3-propanediol and its binary solutions with propanol (I) Dielectric radio spectra”, Z. Fizichesk. Khim., 59,1677-1680 (1985).
17 Nasirzadeh, K., Neueder R., Kunz, W., “Vapor pressures, osmotic and activity coefficientsof electrolytes in protic solvents at different temperatures (2) Lithium bromide in ethanol”, J. Solution Chem., 33,1429-1435 (2004).
18 Safarov, J.T., “Vapor pressures of lithium bromide or lithium chlorideand ethanol solutions”, Fluid Phase Equilib., 243, 38-44 (2006).
19 Zafarani-Moattar, M.T., Aria, M., “Isopiestic determination of osmotic and activity coefficients for solutions of LiCl, LiBr, and LiNO3in 2-propanol at 25 °C”, J. Solution Chem., 30, 351-359 (2001).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
