Analysis of Sucrose Esters with Long Acyl Chain by Coupling ofHPLC-ELSD with ESI-MS System*
ZHU Jinli (朱金麗), TANG Yanfeng (湯艷峰),** , LI Jianhua (李建華) and ZHANG Shufen (張淑芬)
?
Analysis of Sucrose Esters with Long Acyl Chain by Coupling ofHPLC-ELSD with ESI-MS System*
ZHU Jinli (朱金麗)1, TANG Yanfeng (湯艷峰)1,**, LI Jianhua (李建華)1and ZHANG Shufen (張淑芬)2
1School of Chemistry and Chemical Engineering, Nantong University, Nantong 226007, China2State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116012, China
The analysis of sucrose esters with long acyl chain by improved high performance liquid chromatographic method with evaporative light scattering detection (HPLC-ELSD) and electrospray ionization mass spectrum (ESI-MS) is investigated. The improved HPLC-ELSD method for the separation and quantitation of commercial and synthesized sucrose esters is described. Samples are analyzed by means of a reversed-phase (RP) HPLC using a Hypersil C8 column (250 mm×4.6 mm, 5 μm particle size) with methanol-tetrahydrofuran (volume ratio of 90︰10) and water under gradient condition as the mobile phase, in which the flow rate is 1.0 ml·min-1and the column temperature is set at 40°C. This procedure provides a complete separation and determination of monoester, diester, triester and higher esters with different acyl chain lengths in each fraction by a single run, in combination with the ESI-MS technology. With this method, it is possible to determine the approximate compositions of mono- to polyesters in one analysis and quantitate pure positional isomers precisely using an external standard method. It is found that the method of ESI-MS coupling with HPLC system for the analysis of sucrose esters is straight forward, rapid and inexpensive, and can be readily applied in synthesis, puri?cation and structure studies.
sucrose esters, long acyl chain, analysis, high performance liquid chromatographic method with evaporative light scattering detection, electrospray ionization mass spectrum
1 INTRODUCTION
With an increasing concern about environment and natural resources, researches and development of surfactants focus on environmental friendly procedures and natural reproducible resources. Sucrose esters (SE) have been the subject investigated intensively for decades, as they can be produced from renewable, inexpensive, and inexhaustible natural resources. They are widely employed as biodegradable, nontoxic, skin-compatible additives in cosmetics, pharmaceuticals and foods [1-4]. In recent years, application of SE in preparation of nanoparticles is widely studied also [5, 6].
Since the first paper about SE prepared by dimethylformamide (DMF) process published in 1956 [7], there have been several classic methods for the production of SE,.., transparent emulsion process [8], water process [9], solvent-free process [10] and enzyme process [11]. The preparation of SE by these transesterification methods yields a complicated mixture of unreacted sucrose, sucrose monoesters (SME), diesters (SDE) and polyesters (SPE) with fatty acyl groups of various chain lengths and other residues and impurities. Even the acylation of sucrose with a single fatty acid can, theoretically, yield 255 possible isomers from mono- to octaesters [12]. Generally, industrial fatty acid esters in the preparation of SE are mixture of fatty acid esters with different lengths of acyl chains which makes the separation and identification of SE more complicated.
In order to separate and identify different fractions, some methods have hitherto been developed. The most common method of analysis is based on thin layer chromatography (TLC), which gives the ratio of mono-, di-, and higher esters [13]. Gas chromatographic (GC) and gas chromatographic-mass spectrometric (GC-MS) methods were used for quantitative and qualitative analysis after derivatization [14, 15]. As for fast and quantitative methods of analysis, HPLC with UV detection and HPLC with refractive index (RI) detection were used for the separation of different fractions of SE [16, 17]. These methods are either inadequate for the separation of positional isomers or necessary to separately elute SME and SDE using different polar mobile phases. More recently, a gradient high performance liquid chromatographic method with evaporative light scattering detection (HPLC-ELSD) was used to separate SE [18-21]. Moh. [18] reported the separation of SE isomers with long-chain, but only sucrose mono- and dieters were separated and polyesters were retained in the column. Wang. [19, 20] also applied this method in the analysis of SE with short acyl chain. However, the HPLC-ELSD method is not adequate for the characterization of SE only.
In this study an improved reversed-phase high performance liquid chromatography (RP-HPLC) methodis used with a C8 column and an improved mobile phase (methanol-tetrahydrofuran, volume ratio of 90︰10, and water) for the separation of SE with long acyl chain. The sucrose mono-, di- and higher esters are detected using an evaporative light scattering detector (ELSD) by a single run using gradient elution, in combination with the electrospray ionization mass spectrum (ESI-MS) technology. An easy and rapid method for analysis of sucrose esters with different positional isomers in each fraction is also developed to meet all requirements of qualitative and quantitative analytical procedures.
2 EXPERIMENTAL
2.1 Chemicals and reagents
HPLC grade tetrahydrofuran and methanol were purchased from Shenyang Chemical Reagents Factory (Shenyang, China). Water was obtained by distillation of deionized water. Commercial SE were obtained from Mitsubishi-Kagaku Foods Corp., Tokyo, Japan (Ryoto S1670, S1570, S1170 and S970).
2.2 Improved solvent-free synthesis of crude sucrose esters
100.00 g powdered white granulated sucrose, 69.71 g methyl stearate, 2.55 g anhydrous potassium carbonate and 25.46 g potassium stearate were added to a 500 ml three-necked flask. The mixture was heatedwith stirring for about 3 hours at 130-140°C and 664.5 Pa. When the reaction was over, the crude SE were obtained. TLC [toluene-ethyl acetate-methanol-water (10︰5︰4.5︰0.2, by volume) as developing solvent and urea-phosphoric acid-butanol as detection reagent] analysis indicated that the mixture comprised 62% sucrose stearate, 25% soaps and fatty acids, 12% unreacted sucrose and 1% other constituents.
2.3 Purification of SE
The purification of SE followed the procedure described in Ref. [22]. A 500 ml three-necked flask was charged with 250 ml butanone, 150 ml water and 50.0 g crude SE. The mixture was stirred vigorously at 50°C. After the pH was adjusted to about 6.5, 3.8 g anhydrous calcium chloride was added, and then a precipitate was formed. After the precipitate was filtered out, the remaining filtrate formed an upper organic layer and a lower water layer. The organic layer was dried in a rotary evaporator to yield 31.0 g of SE.
2.4 Characterization of SE
2.4.1
The HPLC system consisted of the following components: a Hewlett-Packard Model 1050 series, equipped with an autosampler, an Alltech 2000 evaporative light scattering detector (ELSD), and Agilent chemstation. The chromatographic separation was performed using gradient elution. The mobile phase components were methanol-tetrahydrofuran/water, and delivered at a flow rate of 1.0 ml·min-1. The separation was carried out at 40°C, on a reversed-phase C8 column (250 mm′4.6 mm, 5 μm particle size) purchased from Elite Analytical Instruments Co., Ltd. (Dalian, China). All injections were 10 μl in volume. Solvent gradient conditions are reported in Table 1. The column ef?uent was directed to ELSD. The eluent was nebulized in the ELSD by a stream of dried air using an air compressor at a ?ow rate of 2.4 L·min-1. The nebulization was performed at room temperature and the nebulized ef?uents was evaporated at 80°C. An external standard method is used for the quantitation of samples.
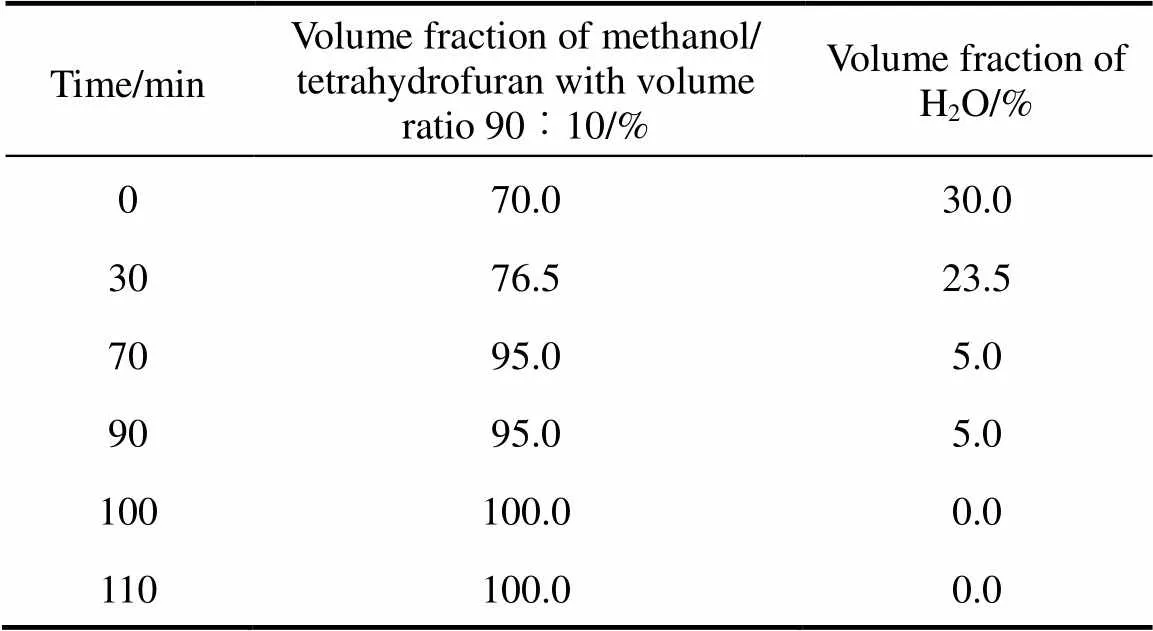
Table 1 Gradient elution program
2.4.2
The components of SE were identified with a quadrupole HP-1100 MS system in the electrospray positive mode. The method was described in Ref. [23]. The mass ions (/) were recorded in a full scan mode with a mass range of 200-1600. In positive or negative mode, the ion source conditions were as follows: ion-spray voltage: +3.5 kV; collision-induced degradation voltage: +50 V. The sample solutions (10 mg·ml-1) used for ESI-MS analysis were prepared by dissolving SE in tetrahydrofuran/methanol (80︰20, by volume). The sample was infused directly into the ion source at a flow rate of 0.2 ml·min-1. Nitrogen was used as a drying gas at a flow rate of 5.0 L·min-1. The temperature of drying gas was 350°C, and the nebulizer pressure was 137.86 kPa.
3 RESULTS AND DISCUSSION
3.1 The improved HPLC-ELSD conditions
We ever tried to use reproduce the HPLC method for the separation of sucrose monoesters and sucrose diesters of commercial SE as described by Moh. [18], but we were not able to obtain anticipated results. In Moh’s method, the binary gradient consists initially of 75% methanol and 25% water. After 70 min, the ratio is changed to 95% methanol and 5% water. This procedure provides a separation of sucrose monoesters and sucrose diesters. However, we found that it is difficult to obtain the reproducible results for two injections of one sample under the identical conditions.
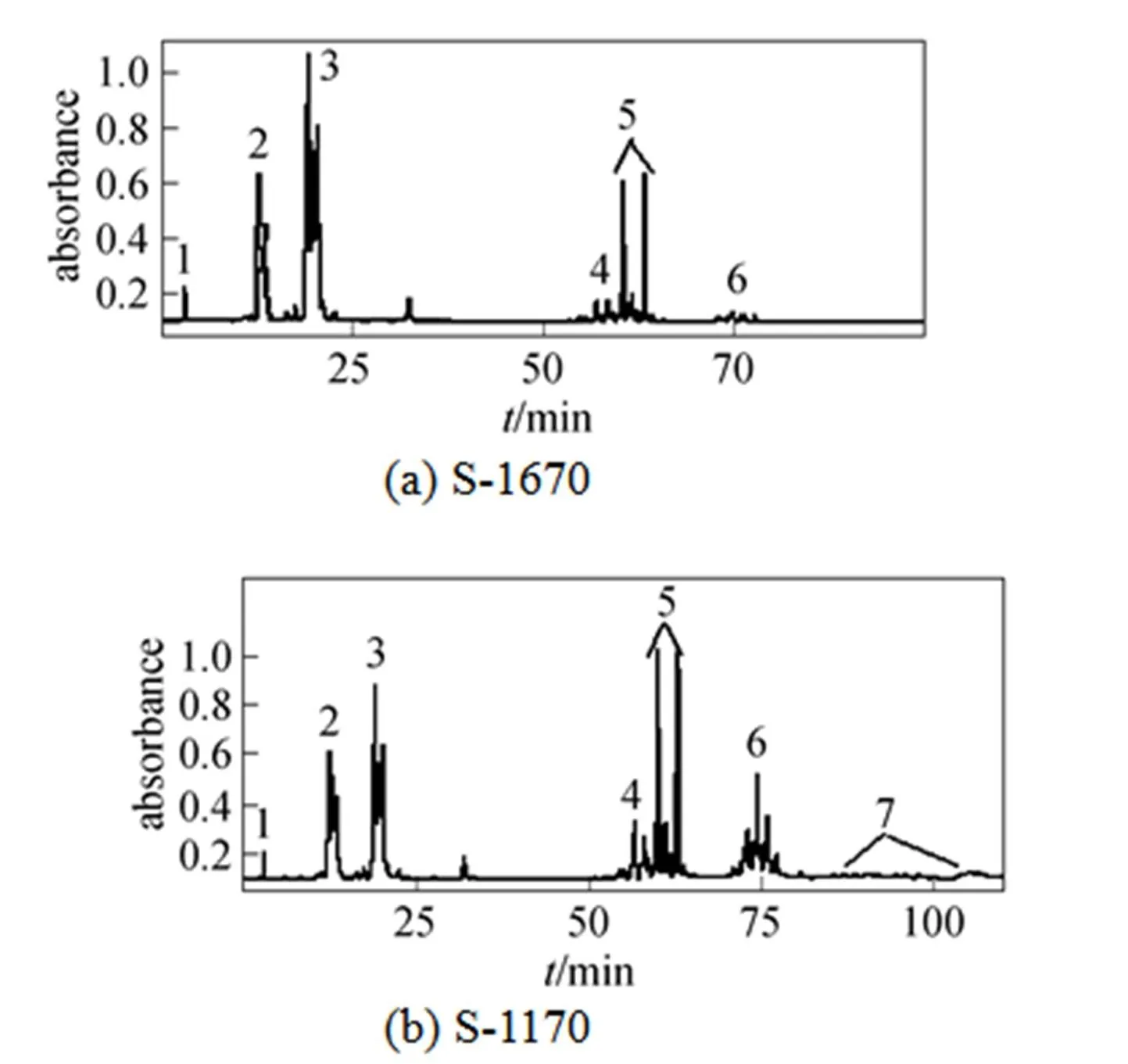
Figure 1 Reversed-phase HPLC separation of S-1670and S-1170
1—sucrose; 2—sucrose monopalmitate; 3—sucrose monostearate; 4—sucrose dipalmitate; 5—sucrose distearate; 6—sucrose triester containing palmitic and stearic acid moieties; 7—sucrose tetraester containing palmitic and stearic acid moieties
In this study, after sucrose monoesters and sucrose diesters were eluted, a mixture of methanol and tetrahydrofuran (90︰10, by volume) was used as eluent and several groups of peaks with high intensity were detected. This result revealed that parts of sucrose diesters and all higher esters were retained in the column, resulting in errors. Higher esters are less polar due to a greater substitution of hydroxyl groups of the sucrose molecule. In order to avoid the retention of sucrose diesters and higher esters and obtain accurate results, less polar mobile phase and highly polar column are needed. By using methanol-tetrahydrofuran and water as the mobile phase instead of methanol and water, and using a Hypersil C8 column instead of C18 column, under the new solvent gradient condition (Table 1), two groups of sucrose monoesters and diesters were identified, which were similar to those reported by Moh. [18], and an additional group of peaks belonging to S1670 or S1170 was also detected by ELSD (Fig. 1). These peaks are not reported by previous studies. Based on the ESI-MS data of S1670 (Fig. 2) and S1170 (Fig. 3), this group of peaks should be identified as sucrose triesters or tetraesters.
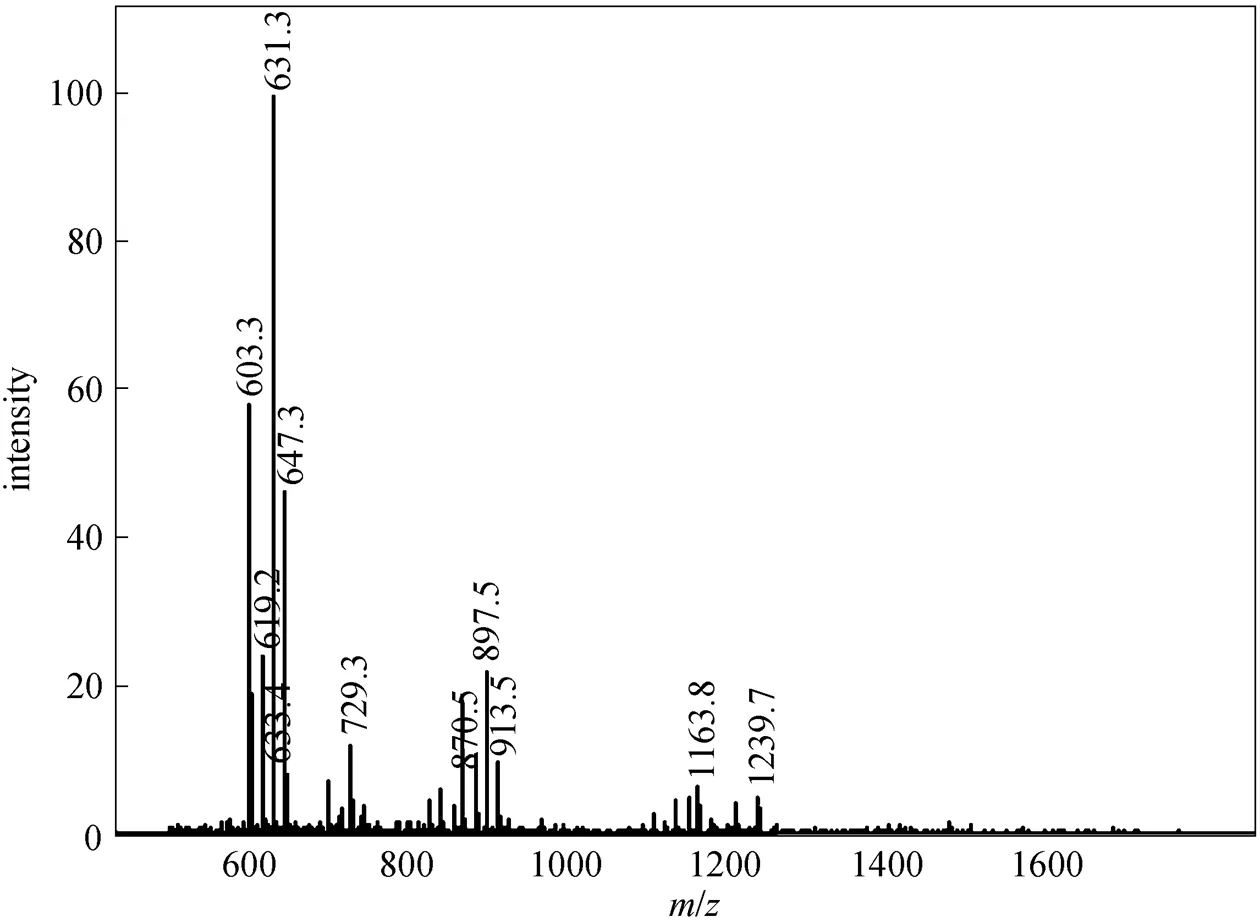
Figure 2 Mass spectrum of S1670 (positive mode)
Figure 3 Mass spectrum of S1170 (positive mode)
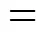
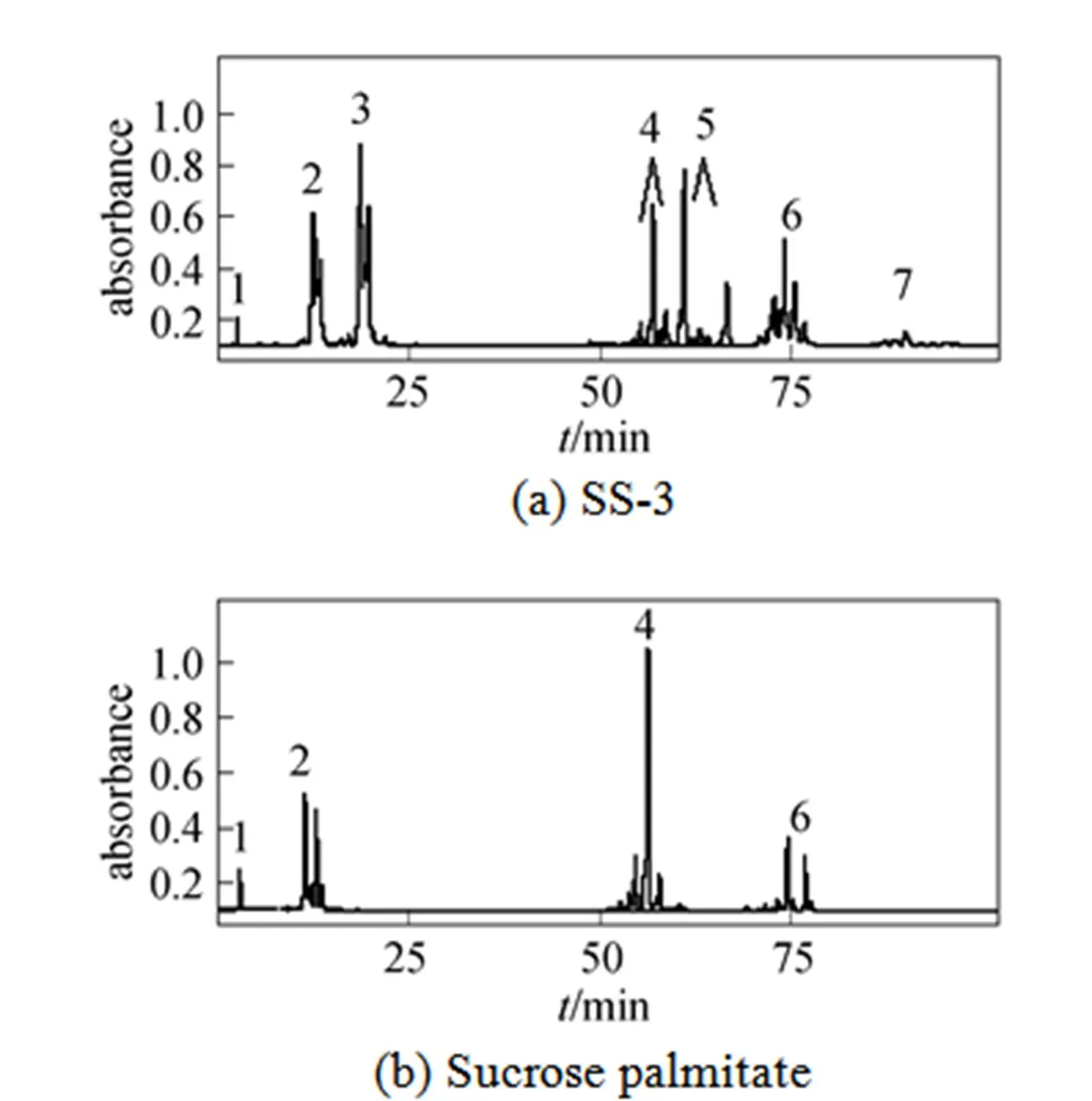
Figure 4 Reversed-phase HPLC separation of SS-3 and sucrose palmitate
1—sucrose; 2—sucrose monopalmitate; 3—sucrose monostearate; 4—sucrose dipalmitate; 5—sucrose distearate; 6—sucrose triester containing palmitic and stearic acid moieties; 7—sucrose tetraester containing palmitic and stearic acid moieties
Commercial SE S1670 and S1170 are analyzed by means of a RP-HPLC using a Hypersil C8 column and methanol-tetrahydrofuran (90︰10, by volume) and water as the mobile phase under improved gradient conditions. This procedure provides a complete separation and determination of monoester, diester, triester and tetraesters with different acyl chain lengths in each fraction by a single run, in combination with the ESI-MS technology.
3.2 Separation and identification of synthesized SE with long acyl chain
The chromatograms of the sucrose stearates (SS-3) synthesized by the improved solvent-free process are shown in Fig. 4 (a). The peaks are similar to those of commercial SE. To further identify the several groups of peaks, sucrose palmitate without stearic acid moieties is prepared and its reversed-phase chromatograms are shown in Fig. 4 (b). Peak 1 is attributed to sucrose according to the standard compound injected. Groups of peaks 2 and 3 are sucrose monopalmitate and sucrose monostearate, respectively. Groups of peaks 4 and 5 are sucrose dipalmitate and sucrose distearate, respectively. Group of peaks 6 in Fig. 4 (b) is sucrose tripalmitate and group of peaks 6 in Fig. 4 (a) is mixtures of sucrose tripalmitate, sucrose tristearate and sucrose triester containing palmitic and stearic acid moieties. Group of peaks 7 is sucrose tetrapalmitate, sucrose tetrastearate and sucrose tetraester containing palmitic and stearic acid moieties.
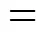
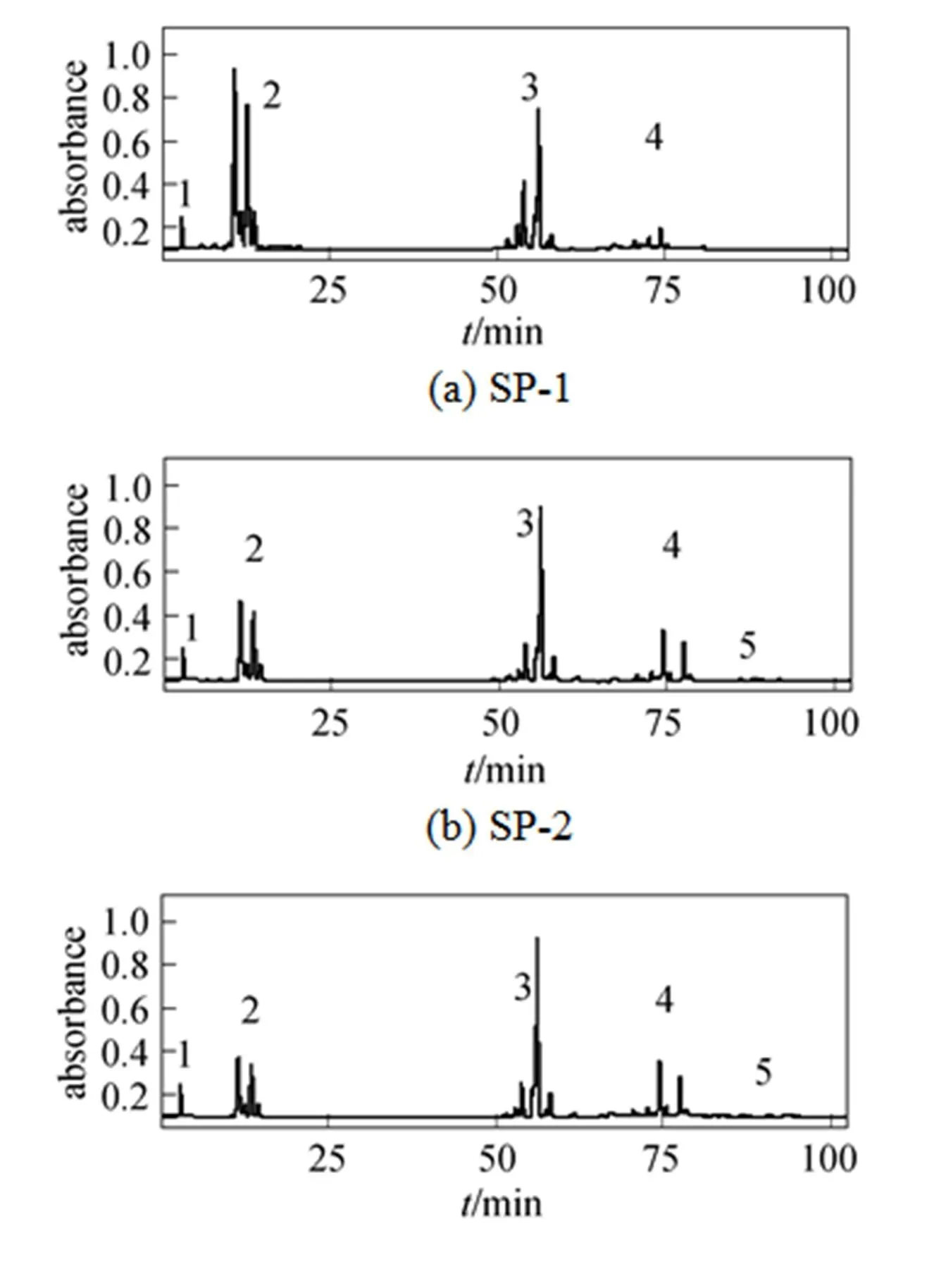
(c) SP-3
Figure 5 Reversed-phase HPLC separation of sucrose palmitates: (a) SP-1, (b) SP-2 and (c) SP-3
1— sucrose; 2—sucrose monopalmitate; 3—sucrose dipalmitate; 4—sucrose tripalmitate; 5—sucrose tetrapalmitate
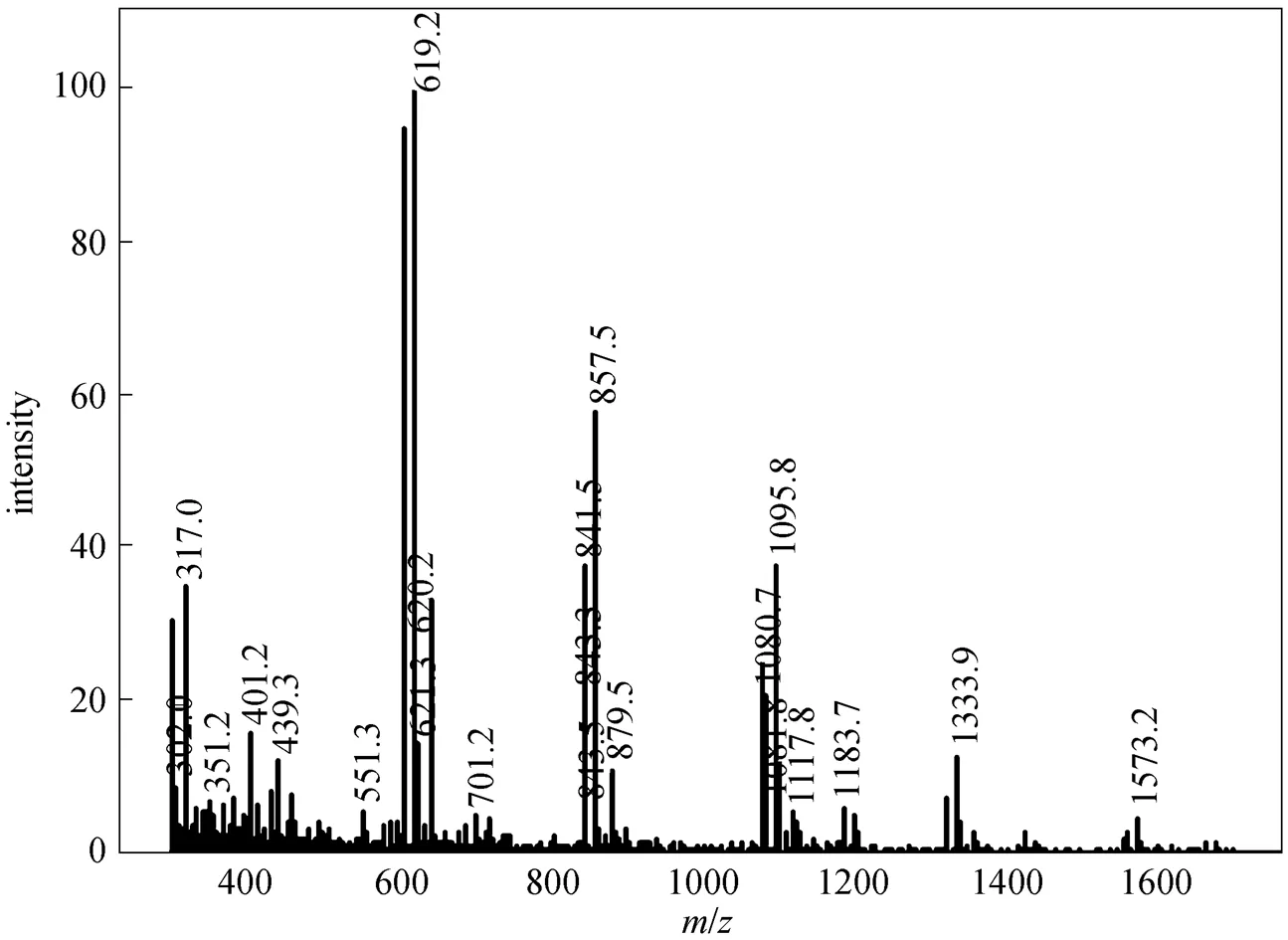
Figure 6 Mass spectrum of SP-3
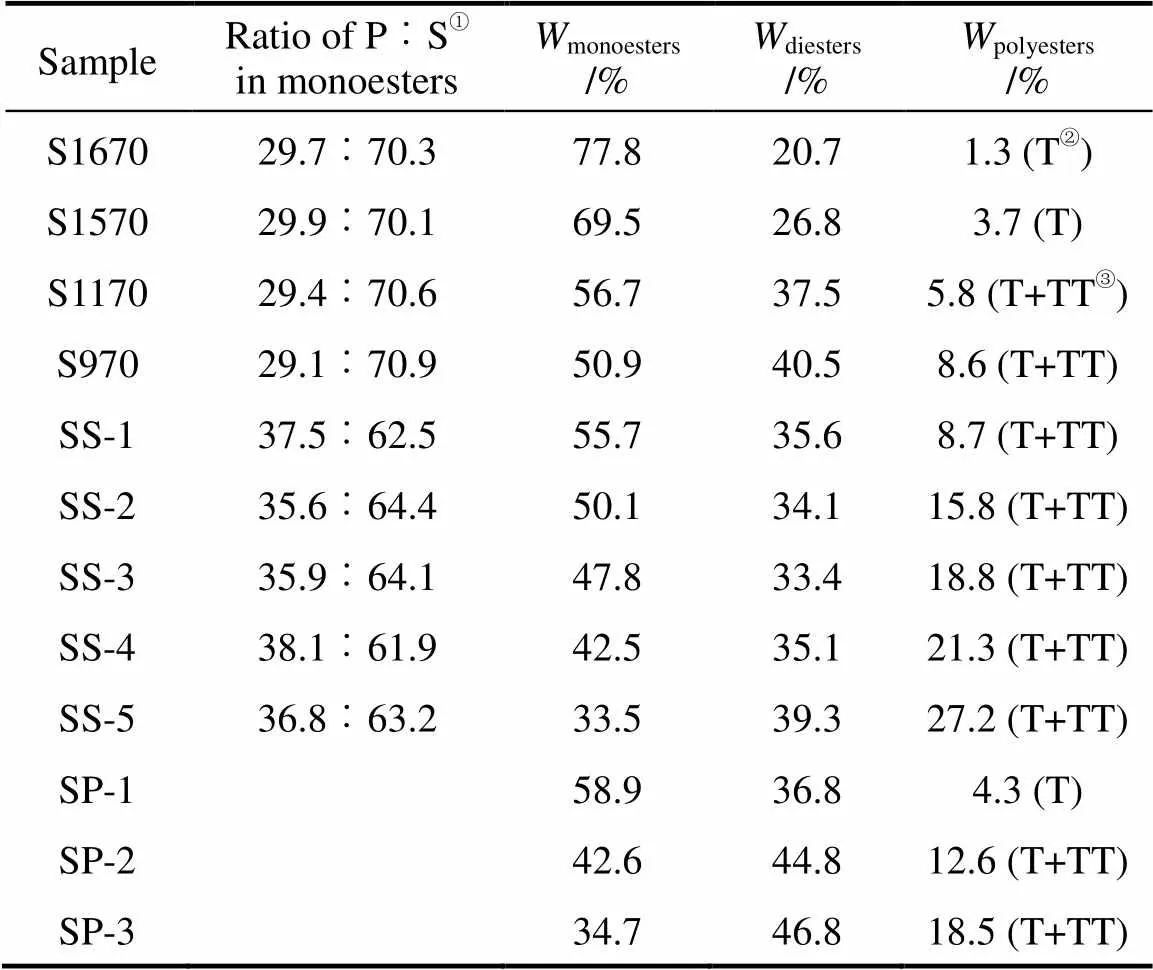
Table 2 Ratio of monopalmitate to monostearate andcomponents of SE analyzed by HPLC and ES-MS
①P—palmitate, S—stearate.②T—triester.③TT—tetraester; T+TT means polyesters contain tri- and tetraesters.
The results from the above analysis give estimate of the ratio between monopalmitate and monostearate, as shown in Table 2 which also gives the percentage of monoester, diester and polyester of commercial and synthesized SE. In contrast to Moh’s method, by combining HPLC-ELSD and ESI-MS system, not only sucrose monoester and diester are separated, but also sucrose triester and sucrose tetraester are separated and identified.
4 CONCLUSIONS
A new method for the separation and identification of SE with long acyl chain was developed based on coupling of ESI-MS with HPLC technology. In this method, improved RP-HPLC conditions of a Hypersil C8 column, methanol-tetrahydrofuran (90︰10, by volume) and water as eluent, and new solvent gradient conditions as the mobile phase were adopted. This procedure provides a complete separation and determination of monoester, diester, triester and higher esters with different acyl chain lengths in each fraction by a single run. It is found that the method coupling of ESI-MS with HPLC system for the analysis of SE with long acyl chain is straightforward, rapid and inexpensive, and can be readily applied for the analysis of SE in synthesis, puri?cation, and structure studies.
1 Sangnark, A., Noomhorm, A., “Effect of dietary fiber from sugarcane bagasse and sucrose ester on dough and bread properties”,, 37 (7), 697-704 (2004).
2 Cázares-Delgadillo, J., Naik, A., Kalia, Y.N., “Skin permeation enhancement by sucrose esters: A pH-dependent phenomenon”,, 297 (1-2), 204-212 (2005).
3 Yamauchi, N., Tokuhara, Y., Ohyama, Y., “Inhibitory effect of sucrose laurate ester on degreening in Citrus nagato-yuzukichi fruit during storage”,, 47 (3), 333-337 (2008).
4 Simonovska, B., Srbinoska, M., Vovk, I., “Analysis of sucrose esters-insecticides from the surface of tobacco plant leaves”,, 1127 (1-2), 273-277 (2006).
5 Asim, N., Radiman, S., Bin Yarmo, M.A., “Synthesis of WO3in nanoscale with the usage of sucrose ester microemulsion and CTAB micelle solution”,, 61 (13), 2652-2657 (2007).
6 Khiew, P.S., Radiman, S., Huang, N.M., “Studies on the growth and characterization of CdS and PbS nanoparticles using sugar-ester nonionic water-in-oil microemulsion”,, 254 (1-2), 235-243 (2003).
7 Osipow, L., Snell, F.D., York, W.C., Finchler, A., “Methods of preparation-fatty acid esters of sucrose”,, 48, 1459-1462 (1956).
8 Osipow, L., Rosenblatt, W., Snell, F.D., “Micro-emulsion preparation of sucrose esters ”,, 44 (5), 307-309 (1967).
9 Fumiaki, Y., Funio, E., Hideki, O., “Process for synthesizing sucrose esters of fatty acids”, U.S.Pat., 3792041 (1974).
10 Feuge, R.O., Zeringue, H.J., Weiss, T. J., “Preparation of sucrose esters by interesterification”,, 47 (2), 56-60 (1970).
11 Hajime, E., suyoshi, U., “Enzymatic synthesis of carbohydrate of fatty acid (I) Esterification of sucrose, glucose, fructose and sorbitol”,, 61 (11), 1761-1765 (1984).
12 Torres, M.C., Dean, M.A., Wagner, F.W., “Chromatographic separations of sucrose, monostearate structure isomers”,, 522, 245-253 (1990).
13 Li, Y.K., Zhang, S.F., Yang, J.Z., “Analysis of sucrose esters by thin-layer chromatography”,, 20 (5), 476-478 (2002). (in Chinese)
14 Gupta, R.K., James, K., Smith, F.J., “Analysis of sucrose dono- and diesters prepared from triglycerides containing C12-C18fatty acids”,, 60,1908-1913 (1983).
15 Karrer, H., Herberg, H., “Analysis of sucrose fatty acid esters by high temperature gas chromatography”,, 15, 585-589 (1992).
16 Kaufman, V.R., Garti, N., “Analysis of sucrose esters composition by HPLC”,, 7, 1195-1205 (1981).
17 Jaspers, M.E.A.P., van Leewen, F.F., Nieuwenhuls, H.J.W., Vianen, G.M., “High performance liquid chromatographic separation of sucrose fatty acid esters”,, 64, 1020-1025 (1987).
18 Moh, M.H., Tang, T.S., Tan, G.H., “Improved separation of sucrose ester isomers using gradient high performance liquid chromatography with evaporative light scattering detection”,, 69, 105-110 (2000).
19 Wang, Q.H., Zhang, S.F., Zhang, P., “Separation and quantitation of sucrose esters using HPLC with evaporative light scattering detection”,, 29, 2399-2412 (2006).
20 Wang, Q.H., Zhang, S.F., Yang, J.Z., “HPLC analysis of sucrose ester analogs using evaporative light scattering detection”,, 30, 2395-2407 (2007).
21 Breda, S., Marija S., Irena, V., “Analysis of sucrose esters-insecticides from the surface of tobacco plant leaves”,, 1127, 273-277 (2006).
22 Zhu, J.L., Zhang, S.F., Yang, J.Z., “Study on production of high-monoester sucrose fatty acid esters by improved metallic salt method and alcoholic separation”,,,, 43 (2), 106-110 (2006).
23 Zhu, J.L., Zhang, S.F., Yang, J.Z., “Analysis of the sucrose fatty acid esters by atmospheric-pressure ionization MS with electrospray ionization spectrometry”,,,, 43 (4), 178-183 (2006).
2009-05-01,
2009-09-07.
the National Natural Science Foundation of China (20906052), the Science Foundation of Nantong City Municipality (K2007011, K2008023), the Science Foundation of Nantong University (08R08) and the University Science Research Project of Jiangsu Province (09KJB530008).
** To whom correspondence should be addressed. E-mail: tangyf@ntu.edu.cn
 Chinese Journal of Chemical Engineering2009年6期
Chinese Journal of Chemical Engineering2009年6期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Working Temperature on the Resistance Characteristic of aPleated Stainless Steel Woven Filter*
- The Numerical Simulation of Collapse Pressure and Boundary of the Cavity Cloud in Venturi*
- Performance of Inner-core Supersonic Gas Separation Device with Droplet Enlargement Method*
- Void Fraction Distributions in Cold-gassed and Hot-sparged Three Phase Stirred Tanks with Multi-impeller*
- Kinetics of COD Removal in a Biological Aerated Filter in thePresence of 2,4,6-Trinitrophenol (Picric Acid)*
- Representation of Phase Behavior of Ionic Liquids Using the Equation of State for Square-well Chain Fluids with Variable Range*
